happens to the system’s entropy in this process?
34. What is the change in entropy in an adiabatic process? Does this imply that adiabatic processes are reversible? Can a process be precisely
adiabatic for a macroscopic system?
35. Does the entropy of a star increase or decrease as it radiates? Does the entropy of the space into which it radiates (which has a temperature of
about 3 K) increase or decrease? What does this do to the entropy of the universe?
36. Explain why a building made of bricks has smaller entropy than the same bricks in a disorganized pile. Do this by considering the number of ways
that each could be formed (the number of microstates in each macrostate).
15.7 Statistical Interpretation of Entropy and the Second Law of Thermodynamics: The Underlying Explanation
37. Explain why a building made of bricks has smaller entropy than the same bricks in a disorganized pile. Do this by considering the number of ways
that each could be formed (the number of microstates in each macrostate).
CHAPTER 15 | THERMODYNAMICS 545
Problems & Exercises
if the average gauge pressure is 2.40 × 105 N/m2 (about 35 psi)? (b)
What average force do you exert on the piston, neglecting friction and
15.1 The First Law of Thermodynamics
gravitational force?
1. What is the change in internal energy of a car if you put 12.0 gal of
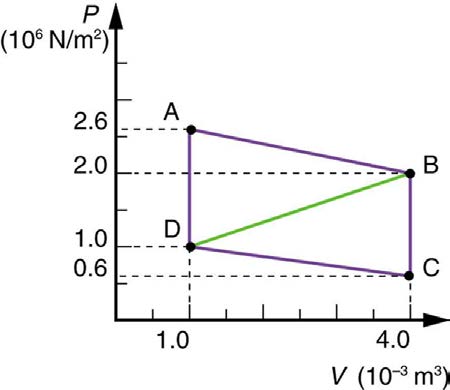
14. Calculate the net work output of a heat engine following path ABCDA
in the figure below.
gasoline into its tank? The energy content of gasoline is 1.3×108 J/gal .
All other factors, such as the car’s temperature, are constant.
2. How much heat transfer occurs from a system, if its internal energy
decreased by 150 J while it was doing 30.0 J of work?
3. A system does 1.80 × 108 J of work while 7.50 × 108 J of heat
transfer occurs to the environment. What is the change in internal energy
of the system assuming no other changes (such as in temperature or by
the addition of fuel)?
4. What is the change in internal energy of a system which does
4.50 × 105 J of work while 3.00 × 106 J of heat transfer occurs into the
system, and 8.00 × 106 J of heat transfer occurs to the environment?
5. Suppose a woman does 500 J of work and 9500 J of heat transfer
occurs into the environment in the process. (a) What is the decrease in
her internal energy, assuming no change in temperature or consumption
of food? (That is, there is no other energy transfer.) (b) What is her
efficiency?
6. (a) How much food energy will a man metabolize in the process of
Figure 15.43
doing 35.0 kJ of work with an efficiency of 5.00%? (b) How much heat
15. What is the net work output of a heat engine that follows path ABDA
transfer occurs to the environment to keep his temperature constant?
in the figure above, with a straight line from B to D? Why is the work
Explicitly show how you follow the steps in the Problem-Solving Strategy
output less than for path ABCDA? Explicitly show how you follow the
for thermodynamics found in Problem-Solving Strategies for
steps in the Problem-Solving Strategies for Thermodynamics.
Thermodynamics.
16. Unreasonable Results
7. (a) What is the average metabolic rate in watts of a man who
What is wrong with the claim that a cyclical heat engine does 4.00 kJ of
metabolizes 10,500 kJ of food energy in one day? (b) What is the
work on an input of 24.0 kJ of heat transfer while 16.0 kJ of heat transfers
maximum amount of work in joules he can do without breaking down fat,
to the environment?
assuming a maximum efficiency of 20.0%? (c) Compare his work output
with the daily output of a 187-W (0.250-horsepower) motor.
17. (a) A cyclical heat engine, operating between temperatures of
8. (a) How long will the energy in a 1470-kJ (350-kcal) cup of yogurt last
450º C and 150º C produces 4.00 MJ of work on a heat transfer of
in a woman doing work at the rate of 150 W with an efficiency of 20.0%
5.00 MJ into the engine. How much heat transfer occurs to the
(such as in leisurely climbing stairs)? (b) Does the time found in part (a)
environment? (b) What is unreasonable about the engine? (c) Which
imply that it is easy to consume more food energy than you can
premise is unreasonable?
reasonably expect to work off with exercise?
18. Construct Your Own Problem
9. (a) A woman climbing the Washington Monument metabolizes
Consider a car’s gasoline engine. Construct a problem in which you
6.00×102 kJ of food energy. If her efficiency is 18.0%, how much heat calculate the maximum efficiency this engine can have. Among the things
transfer occurs to the environment to keep her temperature constant? (b)
to consider are the effective hot and cold reservoir temperatures.
Discuss the amount of heat transfer found in (a). Is it consistent with the
Compare your calculated efficiency with the actual efficiency of car
fact that you quickly warm up when exercising?
engines.
19. Construct Your Own Problem
15.2 The First Law of Thermodynamics and Some
Consider a car trip into the mountains. Construct a problem in which you
Simple Processes
calculate the overall efficiency of the car for the trip as a ratio of kinetic
and potential energy gained to fuel consumed. Compare this efficiency to
10. A car tire contains 0.0380 m3 of air at a pressure of
the thermodynamic efficiency quoted for gasoline engines and discuss
2.20 × 105 N/m2 (about 32 psi). How much more internal energy does why the thermodynamic efficiency is so much greater. Among the factors
this gas have than the same volume has at zero gauge pressure (which
to be considered are the gain in altitude and speed, the mass of the car,
is equivalent to normal atmospheric pressure)?
the distance traveled, and typical fuel economy.
11. A helium-filled toy balloon has a gauge pressure of 0.200 atm and a
15.3 Introduction to the Second Law of
volume of 10.0 L. How much greater is the internal energy of the helium
in the balloon than it would be at zero gauge pressure?
Thermodynamics: Heat Engines and Their Efficiency
12. Steam to drive an old-fashioned steam locomotive is supplied at a
20. A certain heat engine does 10.0 kJ of work and 8.50 kJ of heat
transfer occurs to the environment in a cyclical process. (a) What was the
constant gauge pressure of 1.75 × 106 N/m2 (about 250 psi) to a
heat transfer into this engine? (b) What was the engine’s efficiency?
piston with a 0.200-m radius. (a) By calculating PΔ V , find the work
done by the steam when the piston moves 0.800 m. Note that this is the
21. With 2.56 × 106 J of heat transfer into this engine, a given cyclical
net work output, since gauge pressure is used. (b) Now find the amount
heat engine can do only 1.50 × 105 J of work. (a) What is the engine’s
of work by calculating the force exerted times the distance traveled. Is the
answer the same as in part (a)?
efficiency? (b) How much heat transfer to the environment takes place?
13. A hand-driven tire pump has a piston with a 2.50-cm diameter and a
22. (a) What is the work output of a cyclical heat engine having a 22.0%
maximum stroke of 30.0 cm. (a) How much work do you do in one stroke
efficiency and 6.00 × 109 J of heat transfer into the engine? (b) How
much heat transfer occurs to the environment?
546 CHAPTER 15 | THERMODYNAMICS
23. (a) What is the efficiency of a cyclical heat engine in which 75.0 kJ of
33. A coal-fired electrical power station has an efficiency of 38%. The
heat transfer occurs to the environment for every 95.0 kJ of heat transfer
temperature of the steam leaving the boiler is 550ºC . What percentage
into the engine? (b) How much work does it produce for 100 kJ of heat
of the maximum efficiency does this station obtain? (Assume the
transfer into the engine?
temperature of the environment is 20ºC .)
24. The engine of a large ship does 2.00 × 108 J of work with an
34. Would you be willing to financially back an inventor who is marketing
efficiency of 5.00%. (a) How much heat transfer occurs to the
a device that she claims has 25 kJ of heat transfer at 600 K, has heat
environment? (b) How many barrels of fuel are consumed, if each barrel
transfer to the environment at 300 K, and does 12 kJ of work? Explain
produces 6.00 × 109 J of heat transfer when burned?
your answer.
35. Unreasonable Results
25. (a) How much heat transfer occurs to the environment by an electrical (a) Suppose you want to design a steam engine that has heat transfer to
power station that uses 1.25 × 1014 J of heat transfer into the engine
the environment at 270ºC and has a Carnot efficiency of 0.800. What
with an efficiency of 42.0%? (b) What is the ratio of heat transfer to the
environment to work output? (c) How much work is done?
temperature of hot steam must you use? (b) What is unreasonable about
the temperature? (c) Which premise is unreasonable?
26. Assume that the turbines at a coal-powered power plant were
upgraded, resulting in an improvement in efficiency of 3.32%. Assume
36. Unreasonable Results
that prior to the upgrade the power station had an efficiency of 36% and
Calculate the cold reservoir temperature of a steam engine that uses hot
that the heat transfer into the engine in one day is still the same at
steam at 450ºC and has a Carnot efficiency of 0.700. (b) What is
2.50 × 1014 J . (a) How much more electrical energy is produced due to unreasonable about the temperature? (c) Which premise is
the upgrade? (b) How much less heat transfer occurs to the environment
unreasonable?
due to the upgrade?
27. This problem compares the energy output and heat transfer to the
15.5 Applications of Thermodynamics: Heat Pumps and
environment by two different types of nuclear power stations—one with
Refrigerators
the normal efficiency of 34.0%, and another with an improved efficiency
37. What is the coefficient of performance of an ideal heat pump that has
of 40.0%. Suppose both have the same heat transfer into the engine in
heat transfer from a cold temperature of −25.0ºC to a hot temperature
one day, 2.50 × 1014 J . (a) How much more electrical energy is
of 40.0ºC ?
produced by the more efficient power station? (b) How much less heat
transfer occurs to the environment by the more efficient power station?
38. Suppose you have an ideal refrigerator that cools an environment at
(One type of more efficient nuclear power station, the gas-cooled reactor,
−20.0ºC and has heat transfer to another environment at 50.0ºC .
has not been reliable enough to be economically feasible in spite of its
What is its coefficient of performance?
greater efficiency.)
39. What is the best coefficient of performance possible for a hypothetical
15.4 Carnot’s Perfect Heat Engine: The Second Law of
refrigerator that could make liquid nitrogen at −200ºC and has heat
Thermodynamics Restated
transfer to the environment at 35.0ºC ?
28. A certain gasoline engine has an efficiency of 30.0%. What would the
40. In a very mild winter climate, a heat pump has heat transfer from an
hot reservoir temperature be for a Carnot engine having that efficiency, if
environment at 5.00ºC to one at 35.0ºC . What is the best possible
it operates with a cold reservoir temperature of 200ºC ?
coefficient of performance for these temperatures? Explicitly show how
29. A gas-cooled nuclear reactor operates between hot and cold
you follow the steps in the Problem-Solving Strategies for
Thermodynamics.
reservoir temperatures of 700ºC and 27.0ºC . (a) What is the
maximum efficiency of a heat engine operating between these
41. (a) What is the best coefficient of performance for a heat pump that
temperatures? (b) Find the ratio of this efficiency to the Carnot efficiency
has a hot reservoir temperature of 50.0ºC and a cold reservoir
of a standard nuclear reactor (found in Example 15.4).
temperature of −20.0ºC ? (b) How much heat transfer occurs into the
30. (a) What is the hot reservoir temperature of a Carnot engine that has
warm environment if 3.60×107 J of work ( 10.0kW ⋅ h ) is put into it?
an efficiency of 42.0% and a cold reservoir temperature of 27.0ºC ? (b)
(c) If the cost of this work input is 10.0 cents/kW ⋅ h , how does its cost
What must the hot reservoir temperature be for a real heat engine that
compare with the direct heat transfer achieved by burning natural gas at
achieves 0.700 of the maximum efficiency, but still has an efficiency of
a cost of 85.0 cents per therm. (A therm is a common unit of energy for
42.0% (and a cold reservoir at 27.0ºC )? (c) Does your answer imply
practical limits to the efficiency of car gasoline engines?
natural gas and equals 1.055×108 J .)
31. Steam locomotives have an efficiency of 17.0% and operate with a
42. (a) What is the best coefficient of performance for a refrigerator that
hot steam temperature of 425ºC . (a) What would the cold reservoir
cools an environment at −30.0ºC and has heat transfer to another
temperature be if this were a Carnot engine? (b) What would the
environment at 45.0ºC ? (b) How much work in joules must be done for
maximum efficiency of this steam engine be if its cold reservoir
a heat transfer of 4186 kJ from the cold environment? (c) What is the
temperature were 150ºC ?
cost of doing this if the work costs 10.0 cents per 3.60×106 J (a
32. Practical steam engines utilize 450ºC steam, which is later
kilowatt-hour)? (d) How many kJ of heat transfer occurs into the warm
exhausted at 270ºC . (a) What is the maximum efficiency that such a
environment? (e) Discuss what type of refrigerator might operate
between these temperatures.
heat engine can have? (b) Since 270ºC steam is still quite hot, a
43. Suppose you want to operate an ideal refrigerator with a cold
second steam engine is sometimes operated using the exhaust of the
first. What is the maximum efficiency of the second engine if its exhaust
temperature of −10.0ºC , and you would like it to have a coefficient of
has a temperature of 150ºC ? (c) What is the overall efficiency of the
performance of 7.00. What is the hot reservoir temperature for such a
refrigerator?
two engines? (d) Show that this is the same efficiency as a single Carnot
engine operating between 450ºC and 150ºC . Explicitly show how you
44. An ideal heat pump is being considered for use in heating an
follow the steps in the Problem-Solving Strategies for
environment with a temperature of 22.0ºC . What is the cold reservoir
Thermodynamics.
temperature if the pump is to have a coefficient of performance of 12.0?
CHAPTER 15 | THERMODYNAMICS 547
45. A 4-ton air conditioner removes 5.06 × 107 J (48,000 British thermal
18.0º C lowest temperature? (Part of Q c could be utilized to operate
units) from a cold environment in 1.00 h. (a) What energy input in joules
heat engines or for simply heating the surroundings, but it rarely is.)
is necessary to do this if the air conditioner has an energy efficiency
56. (a) How much heat transfer occurs from 20.0 kg of 90.0º C water
rating ( EER ) of 12.0? (b) What is the cost of doing this if the work costs
placed in contact with 20.0 kg of 10.0º C water, producing a final
10.0 cents per 3.60 × 106 J (one kilowatt-hour)? (c) Discuss whether
temperature of 50.0º C ? (b) How much work could a Carnot engine do
this cost seems realistic. Note that the energy efficiency rating ( EER ) of
with this heat transfer, assuming it operates between two reservoirs at
an air conditioner or refrigerator is defined to be the number of British
constant temperatures of 90.0º C and 10.0º C ? (c) What increase in
thermal units of heat transfer from a cold environment per hour divided by
the watts of power input.
entropy is produced by mixing 20.0 kg of 90.0º C water with 20.0 kg of
10.0º C water? (d) Calculate the amount of work made unavailable by
46. Show that the coefficients of performance of refrigerators and heat
pumps are related by COP
this mixing using a low temperature of 10.0º C , and compare it with the
ref = COP hp − 1 .
work done by the Carnot engine. Explicitly show how you follow the steps
Start with the definitions of the COP s and the conservation of energy
in the Problem-Solving Strategies for Entropy. (e) Discuss how
everyday processes make increasingly more energy unavailable to do
relationship between Q h , Q c , and W .
work, as implied by this problem.
15.6 Entropy and the Second Law of Thermodynamics:
15.7 Statistical Interpretation of Entropy and the Second
Disorder and the Unavailability of Energy
Law of Thermodynamics: The Underlying Explanation
57. Using Table 15.4, verify the contention that if you toss 100 coins each
47. (a) On a winter day, a certain house loses 5.00 × 108 J of heat to
second, you can expect to get 100 heads or 100 tails once in 2 × 1022
the outside (about 500,000 Btu). What is the total change in entropy due
years; calculate the time to two-digit accuracy.
to this heat transfer alone, assuming an average indoor temperature of
21.0º C and an average outdoor temperature of 5.00º C ? (b) This
58. What percent of the time will you get something in the range from 60
large change in entropy implies a large amount of energy has become
heads and 40 tails through 40 heads and 60 tails when tossing 100
unavailable to do work. Where do we find more energy when such
coins? The total number of microstates in that range is 1.22 × 1030 .
energy is lost to us?
(Consult Table 15.4.)
48. On a hot summer day, 4.00 × 106 J of heat transfer into a parked
59. (a) If tossing 100 coins, how many ways (microstates) are there to get
the three most likely macrostates of 49 heads and 51 tails, 50 heads and
car takes place, increasing its temperature from 35.0º C to 45.0º C .
50 tails, and 51 heads and 49 tails? (b) What percent of the total
What is the increase in entropy of the car due to this heat transfer alone?
possibilities is this? (Consult Table 15.4.)
49. A hot rock ejected from a volcano’s lava fountain cools from
60. (a) What is the change in entropy if you start with 100 coins in the 45
1100º C to 40.0º C , and its entropy decreases by 950 J/K. How much heads and 55 tails macrostate, toss them, and get 51 heads and 49 tails?
heat transfer occurs from the rock?
(b) What if you get 75 heads and 25 tails? (c) How much more likely is 51
heads and 49 tails than 75 heads and 25 tails? (d) Does either outcome
50. When 1.60 × 105 J of heat transfer occurs into a meat pie initially at violate the second law of thermodynamics?
20.0º C , its entropy increases by 480 J/K. What is its final temperature? 61. (a) What is the change in entropy if you start with 10 coins in the 5
heads and 5 tails macrostate, toss them, and get 2 heads and 8 tails? (b)
51. The Sun radiates energy at the rate of 3.80 × 1026 W from its
How much more likely is 5 heads and 5 tails than 2 heads and 8 tails?
5500º C surface into dark empty space (a negligible fraction radiates
(Take the ratio of the number of microstates to find out.) (c) If you were
onto Earth and the other planets). The effective temperature of deep
betting on 2 heads and 8 tails would you accept odds of 252 to 45?
Explain why or why not.
space is −270º C . (a) What is the increase in entropy in one day due to
this heat transfer? (b) How much work is made unavailable?
Table 15.5 10-Coin Toss
52. (a) In reaching equilibrium, how much heat transfer occurs from 1.00
Macrostate
Number of Microstates ( W)
kg of water at 40.0º C when it is placed in contact with 1.00 kg of
20.0º C water in reaching equilibrium? (b) What is the change in
Heads
Tails
entropy due to this heat transfer? (c) How much work is made
10
0
1
unavailable, taking the lowest temperature to be 20.0º C ? Explicitly
9
1
10
show how you follow the steps in the Problem-Solving Strategies for
Entropy.
8
2
45
53. What is the decrease in entropy of 25.0 g of water that condenses on
7
3
120
a bathroom mirror at a temperature of 35.0º C , assuming no change in
6
4
210
temperature and given the latent heat of vaporization to be 2450 kJ/kg?
5
5
252
54. Find the increase in entropy of 1.00 kg of liquid nitrogen that starts at
4
6
210
its boiling temperature, boils, and warms to 20.0º C at constant
pressure.
3
7




