• Partial pressure is the pressure a gas would create if it existed alone.
• Dalton’s law states that the total pressure is the sum of the partial pressures of all of the gases present.
13.6 Humidity, Evaporation, and Boiling
• Relative humidity is the fraction of water vapor in a gas compared to the saturation value.
• The saturation vapor density can be determined from the vapor pressure for a given temperature.
• Percent relative humidity is defined to be
percent relative humidity =
vapor density
saturation vapor density×100.
• The dew point is the temperature at which air reaches 100% relative humidity.
Conceptual Questions
13.1 Temperature
1. What does it mean to say that two systems are in thermal equilibrium?
2. Give an example of a physical property that varies with temperature and describe how it is used to measure temperature.
3. When a cold alcohol thermometer is placed in a hot liquid, the column of alcohol goes down slightly before going up. Explain why.
4. If you add boiling water to a cup at room temperature, what would you expect the final equilibrium temperature of the unit to be? You will need to
include the surroundings as part of the system. Consider the zeroth law of thermodynamics.
13.2 Thermal Expansion of Solids and Liquids
5. Thermal stresses caused by uneven cooling can easily break glass cookware. Explain why Pyrex®, a glass with a small coefficient of linear
expansion, is less susceptible.
6. Water expands significantly when it freezes: a volume increase of about 9% occurs. As a result of this expansion and because of the formation and
growth of crystals as water freezes, anywhere from 10% to 30% of biological cells are burst when animal or plant material is frozen. Discuss the
implications of this cell damage for the prospect of preserving human bodies by freezing so that they can be thawed at some future date when it is
hoped that all diseases are curable.
7. One method of getting a tight fit, say of a metal peg in a hole in a metal block, is to manufacture the peg slightly larger than the hole. The peg is
then inserted when at a different temperature than the block. Should the block be hotter or colder than the peg during insertion? Explain your answer.
8. Does it really help to run hot water over a tight metal lid on a glass jar before trying to open it? Explain your answer.
9. Liquids and solids expand with increasing temperature, because the kinetic energy of a body’s atoms and molecules increases. Explain why some
materials shrink with increasing temperature.
13.3 The Ideal Gas Law
10. Find out the human population of Earth. Is there a mole of people inhabiting Earth? If the average mass of a person is 60 kg, calculate the mass
of a mole of people. How does the mass of a mole of people compare with the mass of Earth?
11. Under what circumstances would you expect a gas to behave significantly differently than predicted by the ideal gas law?
12. A constant-volume gas thermometer contains a fixed amount of gas. What property of the gas is measured to indicate its temperature?
13.4 Kinetic Theory: Atomic and Molecular Explanation of Pressure and Temperature
13. How is momentum related to the pressure exerted by a gas? Explain on the atomic and molecular level, considering the behavior of atoms and
molecules.
13.5 Phase Changes
14. A pressure cooker contains water and steam in equilibrium at a pressure greater than atmospheric pressure. How does this greater pressure
increase cooking speed?
15. Why does condensation form most rapidly on the coldest object in a room—for example, on a glass of ice water?
16. What is the vapor pressure of solid carbon dioxide (dry ice) at – 78.5ºC ?
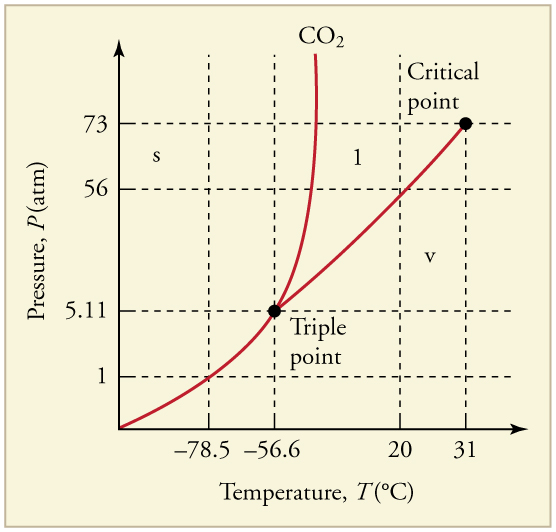
CHAPTER 13 | TEMPERATURE, KINETIC THEORY, AND THE GAS LAWS 465
Figure 13.36 The phase diagram for carbon dioxide. The axes are nonlinear, and the graph is not to scale. Dry ice is solid carbon dioxide and has a sublimation temperature of
– 78.5ºC .
17. Can carbon dioxide be liquefied at room temperature ( 20ºC )? If so, how? If not, why not? (See Figure 13.36.)
18. Oxygen cannot be liquefied at room temperature by placing it under a large enough pressure to force its molecules together. Explain why this is.
19. What is the distinction between gas and vapor?
13.6 Humidity, Evaporation, and Boiling
20. Because humidity depends only on water’s vapor pressure and temperature, are the saturation vapor densities listed in Table 13.5 valid in an atmosphere of helium at a pressure of 1.01×105 N/m2 , rather than air? Are those values affected by altitude on Earth?
21. Why does a beaker of 40.0ºC water placed in a vacuum chamber start to boil as the chamber is evacuated (air is pumped out of the chamber)?
At what pressure does the boiling begin? Would food cook any faster in such a beaker?
22. Why does rubbing alcohol evaporate much more rapidly than water at STP (standard temperature and pressure)?
466 CHAPTER 13 | TEMPERATURE, KINETIC THEORY, AND THE GAS LAWS
Problems & Exercises
reaches 22.0ºC ? (b) How much less water would overflow under the
same conditions?
13.1 Temperature
18. Most automobiles have a coolant reservoir to catch radiator fluid that
may overflow when the engine is hot. A radiator is made of copper and is
1. What is the Fahrenheit temperature of a person with a 39.0ºC fever? filled to its 16.0-L capacity when at 10.0ºC. What volume of radiator
2. Frost damage to most plants occurs at temperatures of 28.0ºF or
fluid will overflow when the radiator and fluid reach their 95.0ºC
lower. What is this temperature on the Kelvin scale?
operating temperature, given that the fluid’s volume coefficient of
3. To conserve energy, room temperatures are kept at 68.0ºF in the
expansion is β = 400 × 10 – 6 / ºC ? Note that this coefficient is
winter and 78.0ºF in the summer. What are these temperatures on the
approximate, because most car radiators have operating temperatures of
Celsius scale?
greater than 95.0ºC.
4. A tungsten light bulb filament may operate at 2900 K. What is its
19. A physicist makes a cup of instant coffee and notices that, as the
Fahrenheit temperature? What is this on the Celsius scale?
coffee cools, its level drops 3.00 mm in the glass cup. Show that this
5. The surface temperature of the Sun is about 5750 K. What is this
decrease cannot be due to thermal contraction by calculating the
temperature on the Fahrenheit scale?
decrease in level if the 350 cm3 of coffee is in a 7.00-cm-diameter cup
6. One of the hottest temperatures ever recorded on the surface of Earth
and decreases in temperature from 95.0ºC to 45.0ºC. (Most of the
was 134ºF in Death Valley, CA. What is this temperature in Celsius
drop in level is actually due to escaping bubbles of air.)
degrees? What is this temperature in Kelvin?
20. (a) The density of water at 0ºC is very nearly 1000 kg/m3 (it is
7. (a) Suppose a cold front blows into your locale and drops the
temperature by 40.0 Fahrenheit degrees. How many degrees Celsius
actually 999.84 kg/m3 ), whereas the density of ice at 0ºC is
does the temperature decrease when there is a 40.0ºF decrease in
temperature? (b) Show that any change in temperature in Fahrenheit
917 kg/m3 . Calculate the pressure necessary to keep ice from
degrees is nine-fifths the change in Celsius degrees.
expanding when it freezes, neglecting the effect such a large pressure
8. (a) At what temperature do the Fahrenheit and Celsius scales have the would have on the freezing temperature. (This problem gives you only an
same numerical value? (b) At what temperature do the Fahrenheit and
indication of how large the forces associated with freezing water might
Kelvin scales have the same numerical value?
be.) (b) What are the implications of this result for biological cells that are
frozen?
13.2 Thermal Expansion of Solids and Liquids
21. Show that β ≈ 3α , by calculating the change in volume Δ V of a
9. The height of the Washington Monument is measured to be 170 m on
cube with sides of length L.
a day when the temperature is 35.0ºC . What will its height be on a day
when the temperature falls to – 10.0ºC ? Although the monument is
13.3 The Ideal Gas Law
made of limestone, assume that its thermal coefficient of expansion is the
same as marble’s.
22. The gauge pressure in your car tires is 2.50×105 N/m2 at a
10. How much taller does the Eiffel Tower become at the end of a day
temperature of 35.0ºC when you drive it onto a ferry boat to Alaska.
when the temperature has increased by 15ºC ? Its original height is 321
What is their gauge pressure later, when their temperature has dropped
m and you can assume it is made of steel.
to – 40.0ºC ?
11. What is the change in length of a 3.00-cm-long column of mercury if
23. Convert an absolute pressure of 7.00×105 N/m2 to gauge
its temperature changes from 37.0ºC to 40.0ºC , assuming the
pressure in lb/in2 . (This value was stated to be just less than
mercury is unconstrained?
12. How large an expansion gap should be left between steel railroad
90.0 lb/in2 in Example 13.9. Is it?)
rails if they may reach a maximum temperature 35.0ºC greater than
24. Suppose a gas-filled incandescent light bulb is manufactured so that
when they were laid? Their original length is 10.0 m.
the gas inside the bulb is at atmospheric pressure when the bulb has a
13. You are looking to purchase a small piece of land in Hong Kong. The
temperature of 20.0ºC . (a) Find the gauge pressure inside such a bulb
price is “only” $60,000 per square meter! The land title says the
when it is hot, assuming its average temperature is 60.0ºC (an
dimensions are 20 m × 30 m. By how much would the total price
approximation) and neglecting any change in volume due to thermal
change if you measured the parcel with a steel tape measure on a day
expansion or gas leaks. (b) The actual final pressure for the light bulb will
when the temperature was 20ºC above normal?
be less than calculated in part (a) because the glass bulb will expand.
What will the actual final pressure be, taking this into account? Is this a
14. Global warming will produce rising sea levels partly due to melting ice
negligible difference?
caps but also due to the expansion of water as average ocean
temperatures rise. To get some idea of the size of this effect, calculate
25. Large helium-filled balloons are used to lift scientific equipment to
the change in length of a column of water 1.00 km high for a temperature
high altitudes. (a) What is the pressure inside such a balloon if it starts
increase of 1.00ºC. Note that this calculation is only approximate
out at sea level with a temperature of 10.0ºC and rises to an altitude
because ocean warming is not uniform with depth.
where its volume is twenty times the original volume and its temperature
is – 50.0ºC ? (b) What is the gauge pressure? (Assume atmospheric
15. Show that 60.0 L of gasoline originally at 15.0ºC will expand to 61.1 pressure is constant.)
L when it warms to 35.0ºC, as claimed in Example 13.4.
26. Confirm that the units of nRT are those of energy for each value of
16. (a) Suppose a meter stick made of steel and one made of invar (an
R : (a) 8.31 J/mol ⋅ K , (b) 1.99 cal/mol ⋅ K , and (c)
alloy of iron and nickel) are the same length at 0ºC . What is their
0.0821 L ⋅ atm/mol ⋅ K .
difference in length at 22.0ºC ? (b) Repeat the calculation for two
30.0-m-long surveyor’s tapes.
27. In the text, it was shown that N / V = 2.68×1025 m−3 for gas at
17. (a) If a 500-mL glass beaker is filled to the brim with ethyl alcohol at a
STP. (a) Show that this quantity is equivalent to
temperature of 5.00ºC, how much will overflow when its temperature
N / V = 2.68×1019 cm−3 , as stated. (b) About how many atoms are
CHAPTER 13 | TEMPERATURE, KINETIC THEORY, AND THE GAS LAWS 467
there in one μm3 (a cubic micrometer) at STP? (c) What does your
40. Average atomic and molecular speeds ( v rms) are large, even at low
answer to part (b) imply about the separation of atoms and molecules?
temperatures. What is v rms for helium atoms at 5.00 K, just one degree
28. Calculate the number of moles in the 2.00-L volume of air in the lungs above helium’s liquefaction temperature?
of the average person. Note that the air is at 37.0ºC (body
41. (a) What is the average kinetic energy in joules of hydrogen atoms on
temperature).
the 5500ºC surface of the Sun? (b) What is the average kinetic energy
of helium atoms in a region of the solar corona where the temperature is
29. An airplane passenger has 100 cm3 of air in his stomach just
6.00×105 K ?
before the plane takes off from a sea-level airport. What volume will the
air have at cruising altitude if cabin pressure drops to
42. The escape velocity of any object from Earth is 11.2 km/s. (a)
7.50×104 N/m2?
Express this speed in m/s and km/h. (b) At what temperature would
oxygen molecules (molecular mass is equal to 32.0 g/mol) have an
30. (a) What is the volume (in km3 ) of Avogadro’s number of sand
average velocity v rms equal to Earth’s escape velocity of 11.1 km/s?
grains if each grain is a cube and has sides that are 1.0 mm long? (b)
43. The escape velocity from the Moon is much smaller than from Earth
How many kilometers of beaches in length would this cover if the beach
and is only 2.38 km/s. At what temperature would hydrogen molecules
averages 100 m in width and 10.0 m in depth? Neglect air spaces
(molecular mass is equal to 2.016 g/mol) have an average velocity v rms
between grains.
equal to the Moon’s escape velocity?
31. An expensive vacuum system can achieve a pressure as low as
1.00×10 – 7 N/m2
44. Nuclear fusion, the energy source of the Sun, hydrogen bombs, and
at 20ºC . How many atoms are there in a cubic
fusion reactors, occurs much more readily when the average kinetic
centimeter at this pressure and temperature?
energy of the atoms is high—that is, at high temperatures. Suppose you
32. The number density of gas atoms at a certain location in the space
want the atoms in your fusion experiment to have average kinetic
above our planet is about 1.00×1011 m−3 , and the pressure is
energies of 6.40×10 – 14 J . What temperature is needed?
2.75×10 – 10 N/m2 in this space. What is the temperature there?
45. Suppose that the average velocity ( v rms) of carbon dioxide
molecules (molecular mass is equal to 44.0 g/mol) in a flame is found to
33. A bicycle tire has a pressure of 7.00×105 N/m2 at a temperature
be 1.05×105 m/s . What temperature does this represent?
of 18.0ºC and contains 2.00 L of gas. What will its pressure be if you let 46. Hydrogen molecules (molecular mass is equal to 2.016 g/mol) have
out an amount of air that has a volume of 100 cm3 at atmospheric
an average velocity v rms equal to 193 m/s. What is the temperature?
pressure? Assume tire temperature and volume remain constant.
47. Much of the gas near the Sun is atomic hydrogen. Its temperature
34. A high-pressure gas cylinder contains 50.0 L of toxic gas at a
would have to be 1.5×107 K for the average velocity v
pressure of 1.40×107 N/m2 and a temperature of 25.0ºC . Its valve
rms to equal
the escape velocity from the Sun. What is that velocity?
leaks after the cylinder is dropped. The cylinder is cooled to dry ice
temperature ( – 78.5ºC) to reduce the leak rate and pressure so that it
48. There are two important isotopes of uranium— 235 U and 238 U ;
can be safely repaired. (a) What is the final pressure in the tank,
these isotopes are nearly identical chemically but have different atomic
assuming a negligible amount of gas leaks while being cooled and that
there is no phase change? (b) What is the final pressure if one-tenth of
masses. Only 235 U is very useful in nuclear reactors. One of the
the gas escapes? (c) To what temperature must the tank be cooled to
techniques for separating them (gas diffusion) is based on the different
reduce the pressure to 1.00 atm (assuming the gas does not change
average velocities v rms of uranium hexafluoride gas, UF6 . (a) The
phase and that there is no leakage during cooling)? (d) Does cooling the
tank appear to be a practical solution?
molecular masses for 235 U UF6 and 238 U UF6 are 349.0 g/mol
35. Find the number of moles in 2.00 L of gas at 35.0ºC and under
and 352.0 g/mol, respectively. What is the ratio of their average
7.41×107 N/m2
velocities? (b) At what temperature would their average velocities differ
of pressure.
by 1.00 m/s? (c) Do your answers in this problem imply that this
36. Calculate the depth to which Avogadro’s number of table tennis balls
technique may be difficult?
would cover Earth. Each ball has a diameter of 3.75 cm. Assume the
space between balls adds an extra 25.0% to their volume and assume
13.6 Humidity, Evaporation, and Boiling
they are not crushed by their own weight.
49. Dry air is 78.1% nitrogen. What is the partial pressure of nitrogen
37. (a) What is the gauge pressure in a 25.0ºC car tire containing 3.60
when the atmospheric pressure is 1.01×105 N/m2 ?
mol of gas in a 30.0 L volume? (b) What will its gauge pressure be if you
50. (a) What is the vapor pressure of water at 20.0ºC ? (b) What
add 1.00 L of gas originally at atmospheric pressure and 25.0ºC ?
percentage of atmospheric pressure does this correspond to? (c) What
Assume the temperature returns to 25.0ºC and the volume remains
percent of 20.0ºC air is water vapor if it has 100% relative humidity?
constant.
(The density of dry air at 20.0ºC is 1.20 kg/m3 .)
38. (a) In the deep space between galaxies, the density of atoms is as
low as 106 atoms/m3 , and the temperature is a frigid 2.7 K. What is
51. Pressure cookers increase cooking speed by raising the boiling
temperature of water above its value at atmospheric pressure. (a) What
the pressure? (b) What volume (in m3 ) is occupied by 1 mol of gas? (c)
pressure is necessary to raise the boiling point to 120.0ºC ? (b) What
If this volume is a cube, what is the length of its sides in kilometers?
gauge pressure does this correspond to?
13.4 Kinetic Theory: Atomic and Molecular Explanation
52. (a) At what temperature does water boil at an altitude of 1500 m
of Pressure and Temperature
(about 5000 ft) on a day when atmospheric pressure is
8.59×104 N/m2 ? (b) What about at an altitude of 3000 m (about
39. Some incandescent light bulbs are filled with argon gas. What is
v rms for argon atoms near the filament, assuming their temperature is
10,000 ft) when atmospheric pressure is 7.00×104 N/m2 ?
2500 K?
468 CHAPTER 13 | TEMPERATURE, KINETIC THEORY, AND THE GAS LAWS
53. What is the atmospheric pressure on top of Mt. Everest on a day
water boil? (c) Is a significantly higher temperature needed to boil water
when water boils there at a temperature of 70.0ºC?
at a greater depth?
54. At a spot in the high Andes, water boils at 80.0ºC , greatly reducing
67. Integrated Concepts
the cooking speed of potatoes, for example. What is atmospheric
To get an idea of the small effect that temperature has on Archimedes’
pressure at this location?
principle, calculate the fraction of a copper block’s weight that is
supported by the buoyant force in 0ºC water and compare this fraction
55. What is the relative humidity on a 25.0ºC day when the air contains
with the fraction supported in 95.0ºC water.
18.0 g/m3 of water vapor?
68. Integrated Concepts
56. What is the density of water vapor in g/m3 on a hot dry day in the
If you want to cook in water at 150ºC , you need a pressure cooker that
desert when the temperature is 40.0ºC and the relative humidity is
can withstand the necessary pressure. (a) What pressure is required for
the boiling point of water to be this high? (b) If the lid of the pressure
6.00%?
cooker is a disk 25.0 cm in diameter, what force must it be able to
57. A deep-sea diver should breathe a gas mixture that has the same
withstand at this pressure?
oxygen partial pressure as at sea level, where dry air contains 20.9%
69. Unreasonable Results
oxygen and has a total pressure of 1.01×105 N/m2 . (a) What is the
(a) How many moles per cubic meter of an ideal gas are there at a
partial pressure of oxygen at sea level? (b) If the diver breathes a gas
pressure of 1.00×1014 N/m2 and at 0ºC ? (b) What is unreasonable
mixture at a pressure of 2.00×106 N/m2 , what percent oxygen should about this result? (c) Which premise or assumption is responsible?
it be to have the same oxygen partial pressure as at sea level?
70. Unreasonable Results
58. The vapor pressure of water at 40.0ºC is 7.34×103 N/m2 . Using (a) An automobile mechanic claims that an aluminum rod fits loosely into
the ideal gas law, calculate the density of water vapor in g/m3 that
its hole on an aluminum engine block because the engine is hot and the
rod is cold. If the hole is 10.0% bigger in diameter than the 22.0ºC rod,
creates a partial pressure equal to this vapor pressure. The result should
be the same as the saturation vapor density at that temperature
at what temperature will the rod be the same size as the hole? (b) What
is unreasonable about this temperature? (c) Wh




