The large-scale effects of radiation on humans can be divided into two categories: immediate effects and long-term effects. Table 32.4 gives the
immediate effects of whole-body exposures received in less than one day. If the radiation exposure is spread out over more time, greater doses are
needed to cause the effects listed. This is due to the body’s ability to partially repair the damage. Any dose less than 100 mSv (10 rem) is called a
low dose, 0.1 Sv to 1 Sv (10 to 100 rem) is called a moderate dose, and anything greater than 1 Sv (100 rem) is called a high dose. There is no
known way to determine after the fact if a person has been exposed to less than 10 mSv.
Table 32.4 Immediate Effects of Radiation (Adults, Whole Body, Single Exposure)
Dose in Sv [2]
Effect
0–0.10
No observable effect.
0.1 – 1
Slight to moderate decrease in white blood cell counts.
0.5
Temporary sterility; 0.35 for women, 0.50 for men.
1 – 2
Significant reduction in blood cell counts, brief nausea and vomiting. Rarely fatal.
2 – 5
Nausea, vomiting, hair loss, severe blood damage, hemorrhage, fatalities.
4.5
LD50/32. Lethal to 50% of the population within 32 days after exposure if not treated.
5 – 20
Worst effects due to malfunction of small intestine and blood systems. Limited survival.
>20
Fatal within hours due to collapse of central nervous system.
Immediate effects are explained by the effects of radiation on cells and the sensitivity of rapidly reproducing cells to radiation. The first clue that a
person has been exposed to radiation is a change in blood count, which is not surprising since blood cells are the most rapidly reproducing cells in
the body. At higher doses, nausea and hair loss are observed, which may be due to interference with cell reproduction. Cells in the lining of the
digestive system also rapidly reproduce, and their destruction causes nausea. When the growth of hair cells slows, the hair follicles become thin and
break off. High doses cause significant cell death in all systems, but the lowest doses that cause fatalities do so by weakening the immune system
through the loss of white blood cells.
The two known long-term effects of radiation are cancer and genetic defects. Both are directly attributable to the interference of radiation with cell
reproduction. For high doses of radiation, the risk of cancer is reasonably well known from studies of exposed groups. Hiroshima and Nagasaki
survivors and a smaller number of people exposed by their occupation, such as radium dial painters, have been fully documented. Chernobyl victims
will be studied for many decades, with some data already available. For example, a significant increase in childhood thyroid cancer has been
observed. The risk of a radiation-induced cancer for low and moderate doses is generally assumed to be proportional to the risk known for high
doses. Under this assumption, any dose of radiation, no matter how small, involves a risk to human health. This is called the linear hypothesis and it
may be prudent, but it is controversial. There is some evidence that, unlike the immediate effects of radiation, the long-term effects are cumulative
and there is little self-repair. This is analogous to the risk of skin cancer from UV exposure, which is known to be cumulative.
There is a latency period for the onset of radiation-induced cancer of about 2 years for leukemia and 15 years for most other forms. The person is at
risk for at least 30 years after the latency period. Omitting many details, the overall risk of a radiation-induced cancer death per year per rem of
exposure is about 10 in a million, which can be written as 10 / 106 rem · y .
1. Values approximate, difficult to determine.
2. Multiply by 100 to obtain dose in rem.
CHAPTER 32 | MEDICAL APPLICATIONS OF NUCLEAR PHYSICS 1155
If a person receives a dose of 1 rem, his risk each year of dying from radiation-induced cancer is 10 in a million and that risk continues for about 30
years. The lifetime risk is thus 300 in a million, or 0.03 percent. Since about 20 percent of all worldwide deaths are from cancer, the increase due to a
1 rem exposure is impossible to detect demographically. But 100 rem (1 Sv), which was the dose received by the average Hiroshima and Nagasaki
survivor, causes a 3 percent risk, which can be observed in the presence of a 20 percent normal or natural incidence rate.
The incidence of genetic defects induced by radiation is about one-third that of cancer deaths, but is much more poorly known. The lifetime risk of a
genetic defect due to a 1 rem exposure is about 100 in a million or 3.3 / 106 rem ⋅ y , but the normal incidence is 60,000 in a million. Evidence of
such a small increase, tragic as it is, is nearly impossible to obtain. For example, there is no evidence of increased genetic defects among the
offspring of Hiroshima and Nagasaki survivors. Animal studies do not seem to correlate well with effects on humans and are not very helpful. For both
cancer and genetic defects, the approach to safety has been to use the linear hypothesis, which is likely to be an overestimate of the risks of low
doses. Certain researchers even claim that low doses are beneficial. Hormesis is a term used to describe generally favorable biological responses to
low exposures of toxins or radiation. Such low levels may help certain repair mechanisms to develop or enable cells to adapt to the effects of the low
exposures. Positive effects may occur at low doses that could be a problem at high doses.
Even the linear hypothesis estimates of the risks are relatively small, and the average person is not exposed to large amounts of radiation. Table 32.5
lists average annual background radiation doses from natural and artificial sources for Australia, the United States, Germany, and world-wide
averages. Cosmic rays are partially shielded by the atmosphere, and the dose depends upon altitude and latitude, but the average is about 0.40
mSv/y. A good example of the variation of cosmic radiation dose with altitude comes from the airline industry. Monitored personnel show an average
of 2 mSv/y. A 12-hour flight might give you an exposure of 0.02 to 0.03 mSv.
Doses from the Earth itself are mainly due to the isotopes of uranium, thorium, and potassium, and vary greatly by location. Some places have great
natural concentrations of uranium and thorium, yielding doses ten times as high as the average value. Internal doses come from foods and liquids
that we ingest. Fertilizers containing phosphates have potassium and uranium. So we are all a little radioactive. Carbon-14 has about 66 Bq/kg
radioactivity whereas fertilizers may have more than 3000 Bq/kg radioactivity. Medical and dental diagnostic exposures are mostly from x-rays. It
should be noted that x-ray doses tend to be localized and are becoming much smaller with improved techniques. Table 32.6 shows typical doses
received during various diagnostic x-ray examinations. Note the large dose from a CT scan. While CT scans only account for less than 20 percent of
the x-ray procedures done today, they account for about 50 percent of the annual dose received.
Radon is usually more pronounced underground and in buildings with low air exchange with the outside world. Almost all soil contains some 226 Ra
and 222 Rn , but radon is lower in mainly sedimentary soils and higher in granite soils. Thus, the exposure to the public can vary greatly, even within
short distances. Radon can diffuse from the soil into homes, especially basements. The estimated exposure for 222 Rn is controversial. Recent
studies indicate there is more radon in homes than had been realized, and it is speculated that radon may be responsible for 20 percent of lung
cancers, being particularly hazardous to those who also smoke. Many countries have introduced limits on allowable radon concentrations in indoor
air, often requiring the measurement of radon concentrations in a house prior to its sale. Ironically, it could be argued that the higher levels of radon
exposure and their geographic variability, taken with the lack of demographic evidence of any effects, means that low-level radiation is less
dangerous than previously thought.
Radiation Protection
Laws regulate radiation doses to which people can be exposed. The greatest occupational whole-body dose that is allowed depends upon the
country and is about 20 to 50 mSv/y and is rarely reached by medical and nuclear power workers. Higher doses are allowed for the hands. Much
lower doses are permitted for the reproductive organs and the fetuses of pregnant women. Inadvertent doses to the public are limited to 1 / 10 of
occupational doses, except for those caused by nuclear power, which cannot legally expose the public to more than 1 / 1000 of the occupational
limit or 0.05 mSv/y (5 mrem/y). This has been exceeded in the United States only at the time of the Three Mile Island (TMI) accident in 1979.
Chernobyl is another story. Extensive monitoring with a variety of radiation detectors is performed to assure radiation safety. Increased ventilation in
uranium mines has lowered the dose there to about 1 mSv/y.
Table 32.5 Background Radiation Sources and Average Doses
Source
Dose (mSv/y)[3]
Source
Australia Germany United States World
Natural Radiation - external
Cosmic Rays
0.30
0.28
0.30
0.39
Soil, building materials
0.40
0.40
0.30
0.48
Radon gas
0.90
1.1
2.0
1.2
Natural Radiation - internal
40 K, 14C, 226Ra
0.24
0.28
0.40
0.29
Medical & Dental
0.80
0.90
0.53
0.40
TOTAL
2.6
3.0
3.5
2.8
To physically limit radiation doses, we use shielding, increase the distance from a source, and limit the time of exposure.
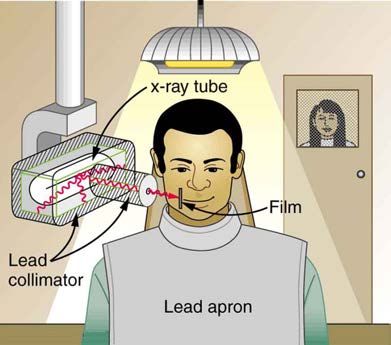
Figure 32.10 illustrates how these are used to protect both the patient and the dental technician when an x-ray is taken. Shielding absorbs radiation and can be provided by any material, including sufficient air. The greater the distance from the source, the more the radiation spreads out. The less
3. Multiply by 100 to obtain dose in mrem/y.
1156 CHAPTER 32 | MEDICAL APPLICATIONS OF NUCLEAR PHYSICS
time a person is exposed to a given source, the smaller is the dose received by the person. Doses from most medical diagnostics have decreased in
recent years due to faster films that require less exposure time.
Figure 32.10 A lead apron is placed over the dental patient and shielding surrounds the x-ray tube to limit exposure to tissue other than the tissue that is being imaged. Fast
films limit the time needed to obtain images, reducing exposure to the imaged tissue. The technician stands a few meters away behind a lead-lined door with a lead glass
window, reducing her occupational exposure.
Table 32.6 Typical Doses Received
During Diagnostic X-ray Exams
Procedure
Effective dose (mSv)
Chest
0.02
Dental
0.01
Skull
0.07
Leg
0.02
Mammogram
0.40
Barium enema 7.0
Upper GI
3.0
CT head
2.0
CT abdomen
10.0
Problem-Solving Strategy
You need to follow certain steps for dose calculations, which are
Step 1. Examine the situation to determine that a person is exposed to ionizing radiation.
Step 2. Identify exactly what needs to be determined in the problem (identify the unknowns). The most straightforward problems ask for a dose
calculation.
Step 3. Make a list of what is given or can be inferred from the problem as stated (identify the knowns). Look for information on the type of radiation,
the energy per event, the activity, and the mass of tissue affected.
Step 4. For dose calculations, you need to determine the energy deposited. This may take one or more steps, depending on the given information.
Step 5. Divide the deposited energy by the mass of the affected tissue. Use units of joules for energy and kilograms for mass. If a dose in Sv is
involved, use the definition that 1 Sv = 1 J/kg .
Step 6. If a dose in mSv is involved, determine the RBE (QF) of the radiation. Recall that 1 mSv = 1 mGy×RBE (or 1 rem = 1 rad×RBE) .
Step 7. Check the answer to see if it is reasonable: Does it make sense? The dose should be consistent with the numbers given in the text for
diagnostic, occupational, and therapeutic exposures.
Example 32.1 Dose from Inhaled Plutonium
Calculate the dose in rem/y for the lungs of a weapons plant employee who inhales and retains an activity of 1.00 μCi of 239 Pu in an
accident. The mass of affected lung tissue is 2.00 kg, the plutonium decays by emission of a 5.23-MeV α particle, and you may assume the
higher value of the RBE for α s from Table 32.2.
Strategy
Dose in rem is defined by 1 rad = 0.01 J/kg and rem = rad×RBE . The energy deposited is divided by the mass of tissue affected and then
multiplied by the RBE. The latter two quantities are given, and so the main task in this example will be to find the energy deposited in one year.

CHAPTER 32 | MEDICAL APPLICATIONS OF NUCLEAR PHYSICS 1157
Since the activity of the source is given, we can calculate the number of decays, multiply by the energy per decay, and convert MeV to joules to
get the total energy.
Solution
The activity R = 1.00 μCi = 3.70×104 Bq = 3.70×104 decays/s. So, the number of decays per year is obtained by multiplying by the
number of seconds in a year:
⎛
⎛
(32.7)
⎝3.70×104 decays/s⎞⎠⎝3.16×107 s⎞⎠ = 1.17×1012 decays.
Thus, the ionizing energy deposited per year is
(32.8)
E = ⎛
⎞
⎛
1.60×10−13 J
⎝1.17×1012 decays⎞⎠⎝5.23 MeV/decay⎞⎠×⎛⎝
MeV
⎠ = 0.978 J.
Dividing by the mass of the affected tissue gives
E
(32.9)
mass = 0.978 J
2.00 kg = 0.489 J/kg.
One Gray is 1.00 J/kg, and so the dose in Gy is
(32.10)
dose in Gy = 0.489 J/kg
1.00 (J/kg)/Gy = 0.489 Gy.
Now, the dose in Sv is
(32.11)
dose in Sv = Gy×RBE
= ⎛
(32.12)
⎝0.489 Gy⎞⎠(20) = 9.8 Sv.
Discussion
First note that the dose is given to two digits, because the RBE is (at best) known only to two digits. By any standard, this yearly radiation dose is
high and will have a devastating effect on the health of the worker. Worse yet, plutonium has a long radioactive half-life and is not readily
eliminated by the body, and so it will remain in the lungs. Being an α emitter makes the effects 10 to 20 times worse than the same ionization
produced by β s, γ rays, or x-rays. An activity of 1.00 µ Ci is created by only 16 µ g of 239 Pu (left as an end-of-chapter problem to verify),
partly justifying claims that plutonium is the most toxic substance known. Its actual hazard depends on how likely it is to be spread out among a
large population and then ingested. The Chernobyl disaster’s deadly legacy, for example, has nothing to do with the plutonium it put into the
environment.
Risk versus Benefit
Medical doses of radiation are also limited. Diagnostic doses are generally low and have further lowered with improved techniques and faster films.
With the possible exception of routine dental x-rays, radiation is used diagnostically only when needed so that the low risk is justified by the benefit of
the diagnosis. Chest x-rays give the lowest doses—about 0.1 mSv to the tissue affected, with less than 5 percent scattering into tissues that are not
directly imaged. Other x-ray procedures range upward to about 10 mSv in a CT scan, and about 5 mSv (0.5 rem) per dental x-ray, again both only
affecting the tissue imaged. Medical images with radiopharmaceuticals give doses ranging from 1 to 5 mSv, usually localized. One exception is the
thyroid scan using 131 I . Because of its relatively long half-life, it exposes the thyroid to about 0.75 Sv. The isotope 123 I is more difficult to produce,
but its short half-life limits thyroid exposure to about 15 mSv.
PhET Explorations: Alpha Decay
Watch alpha particles escape from a polonium nucleus, causing radioactive alpha decay. See how random decay times relate to the half life.
Figure 32.11 Alpha Decay (http://cnx.org/content/m42652/1.3/alpha-decay_en.jar)
32.3 Therapeutic Uses of Ionizing Radiation
Therapeutic applications of ionizing radiation, called radiation therapy or radiotherapy, have existed since the discovery of x-rays and nuclear
radioactivity. Today, radiotherapy is used almost exclusively for cancer therapy, where it saves thousands of lives and improves the quality of life and
longevity of many it cannot save. Radiotherapy may be used alone or in combination with surgery and chemotherapy (drug treatment) depending on
the type of cancer and the response of the patient. A careful examination of all available data has established that radiotherapy’s beneficial effects far
outweigh its long-term risks.

1158 CHAPTER 32 | MEDICAL APPLICATIONS OF NUCLEAR PHYSICS
Medical Application
The earliest uses of ionizing radiation on humans were mostly harmful, with many at the level of snake oil as seen in Figure 32.12. Radium-doped
cosmetics that glowed in the dark were used around the time of World War I. As recently as the 1950s, radon mine tours were promoted as healthful
and rejuvenating—those who toured were exposed but gained no benefits. Radium salts were sold as health elixirs for many years. The gruesome
death of a wealthy industrialist, who became psychologically addicted to the brew, alerted the unsuspecting to the dangers of radium salt elixirs. Most
abuses finally ended after the legislation in the 1950s.
Figure 32.12 The properties of radiation were once touted for far more than its modern use in cancer therapy. Until 1932, radium was advertised for a variety of uses, often
with tragic results. (credit: Struthious Bandersnatch.)
Radiotherapy is effective against cancer because cancer cells reproduce rapidly and, consequently, are more sensitive to radiation. The central
problem in radiotherapy is to make the dose for cancer cells as high as possible while limiting the dose for normal cells. The ratio of abnormal cells
killed to normal cells killed is called the therapeutic ratio, and all radiotherapy techniques are designed to enhance this ratio. Radiation can be
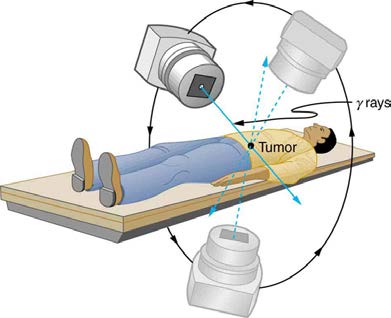
concentrated in cancerous tissue by a number of techniques. One of the most prevalent techniques for well-defined tumors is a geometric technique
shown in Figure 32.13. A narrow beam of radiation is passed through the patient from a variety of directions with a common crossing point in the
tumor. This concentrates the dose in the tumor while spreading it out over a large volume of normal tissue. The external radiation can be x-rays,
60 Co γ rays, or ionizing-particle beams produced by accelerators. Accelerator-produced beams of neutrons, π-mesons , and heavy ions such as
nitrogen nuclei have been employed, and these can be quite effective. These particles have larger QFs or RBEs and sometimes can be better
localized, producing a greater therapeutic ratio. But accelerator radiotherapy is much more expensive and less frequently employed than other forms.
60
Figure 32.13 The
Co source of γ -radiation is rotated around the patient so that the common crossing point is in the tumor, concentrating the dose there. This geometric
technique works for well-defined tumors.
Another form of radiotherapy uses chemically inert radioactive implants. One use is for prostate cancer. Radioactive seeds (about 40 to 100 and the
size of a grain of rice) are placed in the prostate region. The isotopes used are usually 135 I (6-month half life) or 103 Pd (3-month half life). Alpha
emitters have the dual advantages of a large QF and a small range for better localization.
CHAPTER 32 | MEDICAL APPLICATIONS OF NUCLEAR PHYSICS 1159
Radiopharmaceuticals are used for cancer therapy when they can be localized well enough to produce a favorable therapeutic ratio. Thyroid cancer
is commonly treated utilizing radioactive iodine. Thyroid cells concentrate iodine, and cancerous thyroid cells are more aggressive in doing this. An
ingenious use of radiopharmaceuticals in cancer therapy tags antibodies with radioisotopes. Antibodies produced by a patient to combat his cancer
are extracted, cultured, loaded with a radioisotope, and then returned to the patient. The antibodies are concentrated almost entirely in the tissue they
developed to fight, thus localizing the radiation in abnormal tissue. The therapeutic ratio can be quite high for short-range radiation. There is,
however, a significant dose for organs that eliminate radiopharmaceuticals from the body, such as the liver, kidneys, and bladder. As with most
radiotherapy, the technique is limited by the tolerable amount of damage to the normal tissue.
Table 32.7 lists typical therapeutic doses of radiation used against certain cancers. The doses are large, but not fatal because they are localized and spread out in time. Protocols for treatment vary with the type of cancer and the condition and response of the patient. Three to five 200-rem
treatments per week for a period of several weeks is typical. Time between treatments allows the body to repair normal tissue. This effect occurs
because damage is concentrated in the abnormal tissue, and the abnormal tissue is more sensitive to radiation. Damage to normal tissue limits the
doses. You will note that the greatest doses are given to any tissue that is not rapidly reproducing, such as in the adult brain. Lung cancer, on the
other end of the scale, cannot ordinarily be cured with radiation because of the sensitivity of lung tissue and blood to radiation. But radiotherapy for
lung cancer does alleviate symptoms and prolong life and is therefore justified in some cases.
Table 32.7 Cancer Radiotherapy
Type of Cancer
Typical dose (Sv)
Lung
10–20
Hodgkin’s disease
40–45
Skin
40–50
Ovarian
50–75
Breast
50–80+
Brain
80+
Neck
80+
Bone
80+
Soft tissue
80+
Thyroid
80+
Finally, it is interesting to note that chemotherapy employs drugs that interfere with cell division and is, thus, also effective against cancer. It also has
almost the same side effects, such as nausea and hair loss, and risks, such as the inducement of another cancer.
32.4 Food Irradiation
Ionizing radiation is widely used to sterilize medical supplies, such as bandages, and consumer products, such as tampons. Worldwide, it is also
used to irradiate food, an application that promises to grow in the future. Food irradiation is the treatment of food with ionizing radiation. It is used to
reduce pest infestation and to delay spoilage and prevent illness caused by microorganisms. Food irradiation is controversial. Proponents see it as
superior to pasteurization, preservatives, and insecticides, supplanting dangerous chemicals with a more effective process. Opponents see its safety
as unproven, perhaps leaving worse toxic residues as well as presenting an environmental hazard at treatment sites. In developing countries, food
irradiation might increase crop production by 25.0% or more, and reduce food spoilage by a similar amount. It is used chiefly to treat spices and some
fruits, and in some countrie







