e = 0.97 in the infrared. Night-vision scopes can detect the infrared emitted by various warm objects, including humans, and convert it to visible
light.
872 CHAPTER 24 | ELECTROMAGNETIC WAVES
We can examine radiant heat transfer from a house by using a camera capable of detecting infrared radiation. Reconnaissance satellites can detect
buildings, vehicles, and even individual humans by their infrared emissions, whose power radiation is proportional to the fourth power of the absolute
temperature. More mundanely, we use infrared lamps, some of which are called quartz heaters, to preferentially warm us because we absorb infrared
better than our surroundings.
The Sun radiates like a nearly perfect blackbody (that is, it has e = 1 ), with a 6000 K surface temperature. About half of the solar energy arriving at
the Earth is in the infrared region, with most of the rest in the visible part of the spectrum, and a relatively small amount in the ultraviolet. On average,
50 percent of the incident solar energy is absorbed by the Earth.
The relatively constant temperature of the Earth is a result of the energy balance between the incoming solar radiation and the energy radiated from
the Earth. Most of the infrared radiation emitted from the Earth is absorbed by CO2 and H2 O in the atmosphere and then radiated back to Earth
or into outer space. This radiation back to Earth is known as the greenhouse effect, and it maintains the surface temperature of the Earth about
40ºC higher than it would be if there is no absorption. Some scientists think that the increased concentration of CO2 and other greenhouse gases
in the atmosphere, resulting from increases in fossil fuel burning, has increased global average temperatures.
Visible Light
Visible light is the narrow segment of the electromagnetic spectrum to which the normal human eye responds. Visible light is produced by vibrations
and rotations of atoms and molecules, as well as by electronic transitions within atoms and molecules. The receivers or detectors of light largely
utilize electronic transitions. We say the atoms and molecules are excited when they absorb and relax when they emit through electronic transitions.
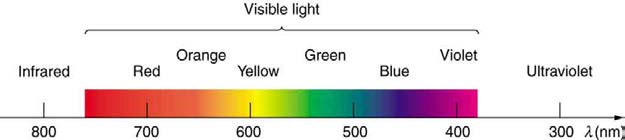
Figure 24.16 shows this part of the spectrum, together with the colors associated with particular pure wavelengths. We usually refer to visible light as having wavelengths of between 400 nm and 750 nm. (The retina of the eye actually responds to the lowest ultraviolet frequencies, but these do not
normally reach the retina because they are absorbed by the cornea and lens of the eye.)
Red light has the lowest frequencies and longest wavelengths, while violet has the highest frequencies and shortest wavelengths. Blackbody
radiation from the Sun peaks in the visible part of the spectrum but is more intense in the red than in the violet, making the Sun yellowish in
appearance.
Figure 24.16 A small part of the electromagnetic spectrum that includes its visible components. The divisions between infrared, visible, and ultraviolet are not perfectly distinct, nor are those between the seven rainbow colors.
Living things—plants and animals—have evolved to utilize and respond to parts of the electromagnetic spectrum they are embedded in. Visible light
is the most predominant and we enjoy the beauty of nature through visible light. Plants are more selective. Photosynthesis makes use of parts of the
visible spectrum to make sugars.
Example 24.3 Integrated Concept Problem: Correcting Vision with Lasers
During laser vision correction, a brief burst of 193-nm ultraviolet light is projected onto the cornea of a patient. It makes a spot 0.80 mm in
diameter and evaporates a layer of cornea 0.30 µ m thick. Calculate the energy absorbed, assuming the corneal tissue has the same properties
as water; it is initially at 34ºC . Assume the evaporated tissue leaves at a temperature of 100ºC .
Strategy
The energy from the laser light goes toward raising the temperature of the tissue and also toward evaporating it. Thus we have two amounts of
heat to add together. Also, we need to find the mass of corneal tissue involved.
Solution
To figure out the heat required to raise the temperature of the tissue to 100ºC , we can apply concepts of thermal energy. We know that
Q
(24.11)
= mcΔ T,
where Q is the heat required to raise the temperature, Δ T is the desired change in temperature, m is the mass of tissue to be heated, and c
is the specific heat of water equal to 4186 J/kg/K.
Without knowing the mass m at this point, we have
Q
(24.12)
= m(4186 J/kg/K)(100ºC – 34ºC) = m(276,276 J/kg) = m(276 kJ/kg).
The latent heat of vaporization of water is 2256 kJ/kg, so that the energy needed to evaporate mass m is
Q
(24.13)
v = mL v = m(2256 kJ/kg).
To find the mass m , we use the equation ρ = m / V , where ρ is the density of the tissue and V is its volume. For this case,
CHAPTER 24 | ELECTROMAGNETIC WAVES 873
m
(24.14)
= ρ V
= (1000 kg/m3)(area × thickness(m3 ))
= (1000 kg/m3)( π(0.80 × 10 – 3 m)2 / 4)(0.30 × 10 – 6 m)
= 0.151 × 10 – 9 kg.
Therefore, the total energy absorbed by the tissue in the eye is the sum of Q and Qv :
(24.15)
Qtot = m(Δ c T + Lv) = (0.151 × 10−9 kg)(276 kJ/kg + 2256 kJ/kg) = 382 × 10−9 kJ.
Discussion
The lasers used for this eye surgery are excimer lasers, whose light is well absorbed by biological tissue. They evaporate rather than burn the
tissue, and can be used for precision work. Most lasers used for this type of eye surgery have an average power rating of about one watt. For our
example, if we assume that each laser burst from this pulsed laser lasts for 10 ns, and there are 400 bursts per second, then the average power
is Qtot × 400 = 150 mW .
Optics is the study of the behavior of visible light and other forms of electromagnetic waves. Optics falls into two distinct categories. When
electromagnetic radiation, such as visible light, interacts with objects that are large compared with its wavelength, its motion can be represented by
straight lines like rays. Ray optics is the study of such situations and includes lenses and mirrors.
When electromagnetic radiation interacts with objects about the same size as the wavelength or smaller, its wave nature becomes apparent. For
example, observable detail is limited by the wavelength, and so visible light can never detect individual atoms, because they are so much smaller
than its wavelength. Physical or wave optics is the study of such situations and includes all wave characteristics.
Take-Home Experiment: Colors That Match
When you light a match you see largely orange light; when you light a gas stove you see blue light. Why are the colors different? What other
colors are present in these?
Ultraviolet Radiation
Ultraviolet means “above violet.” The electromagnetic frequencies of ultraviolet radiation (UV) extend upward from violet, the highest-frequency
visible light. Ultraviolet is also produced by atomic and molecular motions and electronic transitions. The wavelengths of ultraviolet extend from 400
nm down to about 10 nm at its highest frequencies, which overlap with the lowest X-ray frequencies. It was recognized as early as 1801 by Johann
Ritter that the solar spectrum had an invisible component beyond the violet range.
Solar UV radiation is broadly subdivided into three regions: UV-A (320–400 nm), UV-B (290–320 nm), and UV-C (220–290 nm), ranked from long to
shorter wavelengths (from smaller to larger energies). Most UV-B and all UV-C is absorbed by ozone ( O3 ) molecules in the upper atmosphere.
Consequently, 99% of the solar UV radiation reaching the Earth’s surface is UV-A.
Human Exposure to UV Radiation
It is largely exposure to UV-B that causes skin cancer. It is estimated that as many as 20% of adults will develop skin cancer over the course of their
lifetime. Again, treatment is often successful if caught early. Despite very little UV-B reaching the Earth’s surface, there are substantial increases in
skin-cancer rates in countries such as Australia, indicating how important it is that UV-B and UV-C continue to be absorbed by the upper atmosphere.
All UV radiation can damage collagen fibers, resulting in an acceleration of the aging process of skin and the formation of wrinkles. Because there is
so little UV-B and UV-C reaching the Earth’s surface, sunburn is caused by large exposures, and skin cancer from repeated exposure. Some studies
indicate a link between overexposure to the Sun when young and melanoma later in life.
The tanning response is a defense mechanism in which the body produces pigments to absorb future exposures in inert skin layers above living cells.
Basically UV-B radiation excites DNA molecules, distorting the DNA helix, leading to mutations and the possible formation of cancerous cells.
Repeated exposure to UV-B may also lead to the formation of cataracts in the eyes—a cause of blindness among people living in the equatorial belt
where medical treatment is limited. Cataracts, clouding in the eye’s lens and a loss of vision, are age related; 60% of those between the ages of 65
and 74 will develop cataracts. However, treatment is easy and successful, as one replaces the lens of the eye with a plastic lens. Prevention is
important. Eye protection from UV is more effective with plastic sunglasses than those made of glass.
A major acute effect of extreme UV exposure is the suppression of the immune system, both locally and throughout the body.
Low-intensity ultraviolet is used to sterilize haircutting implements, implying that the energy associated with ultraviolet is deposited in a manner
different from lower-frequency electromagnetic waves. (Actually this is true for all electromagnetic waves with frequencies greater than visible light.)
Flash photography is generally not allowed of precious artworks and colored prints because the UV radiation from the flash can cause photo-
degradation in the artworks. Often artworks will have an extra-thick layer of glass in front of them, which is especially designed to absorb UV
radiation.
UV Light and the Ozone Layer
If all of the Sun’s ultraviolet radiation reached the Earth’s surface, there would be extremely grave effects on the biosphere from the severe cell
damage it causes. However, the layer of ozone ( O3 ) in our upper atmosphere (10 to 50 km above the Earth) protects life by absorbing most of the
dangerous UV radiation.
874 CHAPTER 24 | ELECTROMAGNETIC WAVES
Unfortunately, today we are observing a depletion in ozone concentrations in the upper atmosphere. This depletion has led to the formation of an
“ozone hole” in the upper atmosphere. The hole is more centered over the southern hemisphere, and changes with the seasons, being largest in the
spring. This depletion is attributed to the breakdown of ozone molecules by refrigerant gases called chlorofluorocarbons (CFCs).
The UV radiation helps dissociate the CFC’s, releasing highly reactive chlorine (Cl) atoms, which catalyze the destruction of the ozone layer. For
example, the reaction of CFCl3 with a photon of light ( hv) can be written as:
(24.16)
CFCl3 + h v → CFCl2 + Cl.
The Cl atom then catalyzes the breakdown of ozone as follows:
(24.17)
Cl + O3 → ClO + O2 and ClO + O3 → Cl + 2O2.
A single chlorine atom could destroy ozone molecules for up to two years before being transported down to the surface. The CFCs are relatively
stable and will contribute to ozone depletion for years to come. CFCs are found in refrigerants, air conditioning systems, foams, and aerosols.
International concern over this problem led to the establishment of the “Montreal Protocol” agreement (1987) to phase out CFC production in most
countries. However, developing-country participation is needed if worldwide production and elimination of CFCs is to be achieved. Probably the
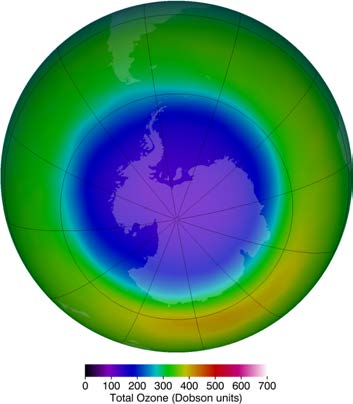
largest contributor to CFC emissions today is India. But the protocol seems to be working, as there are signs of an ozone recovery. (See Figure
24.17.)
Figure 24.17 This map of ozone concentration over Antarctica in October 2011 shows severe depletion suspected to be caused by CFCs. Less dramatic but more general
depletion has been observed over northern latitudes, suggesting the effect is global. With less ozone, more ultraviolet radiation from the Sun reaches the surface, causing
more damage. (credit: NASA Ozone Watch)
Benefits of UV Light
Besides the adverse effects of ultraviolet radiation, there are also benefits of exposure in nature and uses in technology. Vitamin D production in the
skin (epidermis) results from exposure to UVB radiation, generally from sunlight. A number of studies indicate lack of vitamin D can result in the
development of a range of cancers (prostate, breast, colon), so a certain amount of UV exposure is helpful. Lack of vitamin D is also linked to
osteoporosis. Exposures (with no sunscreen) of 10 minutes a day to arms, face, and legs might be sufficient to provide the accepted dietary level.
However, in the winter time north of about 37º latitude, most UVB gets blocked by the atmosphere.
UV radiation is used in the treatment of infantile jaundice and in some skin conditions. It is also used in sterilizing workspaces and tools, and killing
germs in a wide range of applications. It is also used as an analytical tool to identify substances.
When exposed to ultraviolet, some substances, such as minerals, glow in characteristic visible wavelengths, a process called fluorescence. So-called
black lights emit ultraviolet to cause posters and clothing to fluoresce in the visible. Ultraviolet is also used in special microscopes to detect details
smaller than those observable with longer-wavelength visible-light microscopes.
Things Great and Small: A Submicroscopic View of X-Ray Production
X-rays can be created in a high-voltage discharge. They are emitted in the material struck by electrons in the discharge current. There are two
mechanisms by which the electrons create X-rays.
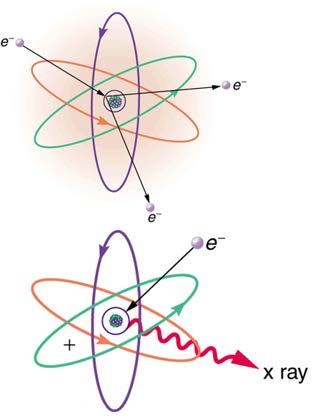
The first method is illustrated in Figure 24.18. An electron is accelerated in an evacuated tube by a high positive voltage. The electron strikes a metal plate (e.g., copper) and produces X-rays. Since this is a high-voltage discharge, the electron gains sufficient energy to ionize the atom.
CHAPTER 24 | ELECTROMAGNETIC WAVES 875
Figure 24.18 Artist’s conception of an electron ionizing an atom followed by the recapture of an electron and emission of an X-ray. An energetic electron strikes an atom
and knocks an electron out of one of the orbits closest to the nucleus. Later, the atom captures another electron, and the energy released by its fall into a low orbit
generates a high-energy EM wave called an X-ray.
In the case shown, an inner-shell electron (one in an orbit relatively close to and tightly bound to the nucleus) is ejected. A short time later,
another electron is captured and falls into the orbit in a single great plunge. The energy released by this fall is given to an EM wave known as an
X-ray. Since the orbits of the atom are unique to the type of atom, the energy of the X-ray is characteristic of the atom, hence the name
characteristic X-ray.
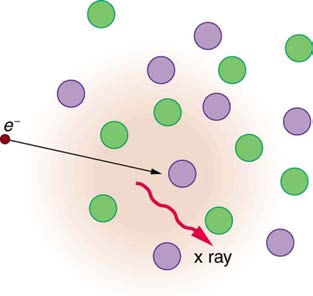
The second method by which an energetic electron creates an X-ray when it strikes a material is illustrated in Figure 24.19. The electron
interacts with charges in the material as it penetrates. These collisions transfer kinetic energy from the electron to the electrons and atoms in the
material.
Figure 24.19 Artist’s conception of an electron being slowed by collisions in a material and emitting X-ray radiation. This energetic electron makes numerous collisions
with electrons and atoms in a material it penetrates. An accelerated charge radiates EM waves, a second method by which X-rays are created.
A loss of kinetic energy implies an acceleration, in this case decreasing the electron’s velocity. Whenever a charge is accelerated, it radiates EM
waves. Given the high energy of the electron, these EM waves can have high energy. We call them X-rays. Since the process is random, a broad
spectrum of X-ray energy is emitted that is more characteristic of the electron energy than the type of material the electron encounters. Such EM
radiation is called “bremsstrahlung” (German for “braking radiation”).
X-Rays
In the 1850s, scientists (such as Faraday) began experimenting with high-voltage electrical discharges in tubes filled with rarefied gases. It was later
found that these discharges created an invisible, penetrating form of very high frequency electromagnetic radiation. This radiation was called an X-
ray, because its identity and nature were unknown.
As described in Things Great and Small, there are two methods by which X-rays are created—both are submicroscopic processes and can be
caused by high-voltage discharges. While the low-frequency end of the X-ray range overlaps with the ultraviolet, X-rays extend to much higher
frequencies (and energies).
X-rays have adverse effects on living cells similar to those of ultraviolet radiation, and they have the additional liability of being more penetrating,
affecting more than the surface layers of cells. Cancer and genetic defects can be induced by exposure to X-rays. Because of their effect on rapidly
dividing cells, X-rays can also be used to treat and even cure cancer.
The widest use of X-rays is for imaging objects that are opaque to visible light, such as the human body or aircraft parts. In humans, the risk of cell
damage is weighed carefully against the benefit of the diagnostic information obtained. However, questions have risen in recent years as to
accidental overexposure of some people during CT scans—a mistake at least in part due to poor monitoring of radiation dose.
The ability of X-rays to penetrate matter depends on density, and so an X-ray image can reveal very detailed density information. Figure 24.20 shows an example of the simplest type of X-ray image, an X-ray shadow on film. The amount of information in a simple X-ray image is impressive, but more
sophisticated techniques, such as CT scans, can reveal three-dimensional information with details smaller than a millimeter.
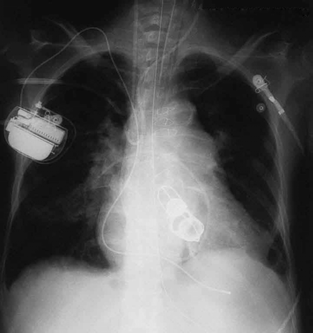
876 CHAPTER 24 | ELECTROMAGNETIC WAVES
Figure 24.20 This shadow X-ray image shows many interesting features, such as artificial heart valves, a pacemaker, and the wires used to close the sternum. (credit: P. P.
Urone)
The use of X-ray technology in medicine is called radiology—an established and relatively cheap tool in comparison to more sophisticated
technologies. Consequently, X-rays are widely available and used extensively in medical diagnostics. During World War I, mobile X-ray units,
advocated by Madame Marie Curie, were used to diagnose soldiers.
Because they can have wavelengths less than 0.01 nm, X-rays can be scattered (a process called X-ray diffraction) to detect the shape of molecules
and the structure of crystals. X-ray diffraction was crucial to Crick, Watson, and Wilkins in the determination of the shape of the double-helix DNA
molecule.
X-rays are also used as a precise tool for trace-metal analysis in X-ray induced fluorescence, in which the energy of the X-ray emissions are related
to the specific types of elements and amounts of materials present.
Gamma Rays
Soon after nuclear radioactivity was first detected in 1896, it was found that at least three distinct types of radiation were being emitted. The most
penetrating nuclear radiation was called a gamma ray ( γ ray) (again a name given because its identity and character were unknown), and it was
later found to be an extremely high frequency electromagnetic wave.
In fact, γ rays are any electromagnetic radiation emitted by a nucleus. This can be from natural nuclear decay or induced nuclear processes in
nuclear reactors and weapons. The lower end of the γ-ray frequency range overlaps the upper end of the X-ray range, but γ rays can have the
highest frequency of any electromagnetic radiation.
Gamma rays have characteristics identical to X-rays of the same frequency—they differ only in source. At higher frequencies, γ rays are more
penetrating and more damaging to living tissue. They have many of the same uses as X-rays, including cancer therapy. Gamma radiation from
radioactive materials is used in nuclear medicine.
Figure 24.21 shows a medical image based on γ rays. Food spoilage can be greatly inhibited by exposing it to large doses of γ radiation, thereby obliterating responsible microorganisms. Damage to food cells through irradiation occurs as well, and the long-term hazards of consuming radiation-preserved food are unknown and controversial for some groups. Both X-ray and γ-ray technologies are also used in scanning luggage at airports.
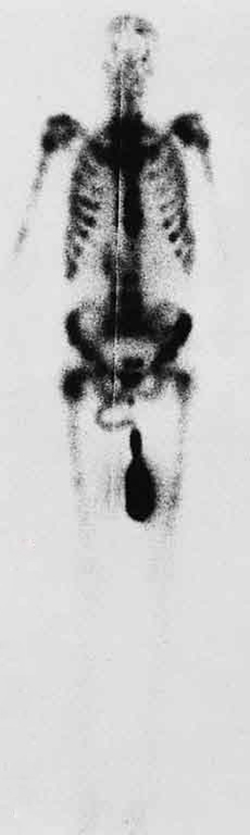

CHAPTER 24 | ELECTROMAGNETIC WAVES 877
Figure 24.21 This is an image of the γ rays emitted by nuclei in a compound that is concentrated in the bones and eliminated through the kidneys. Bone cancer is evidenced by nonuniform concentration in similar structures. For example, some ribs are darker than others. (credit: P. P. Urone)
Detecting Electromagnetic Waves from Space
A final note on star gazing. The entire electromagnetic spectrum is used by researchers for investigating stars, space, and time. As noted earlier,
Penzias and Wilson detected microwaves to identify the background radiation originating from the Big Bang. Radio telescopes such as the Arecibo
Radio Telescope in Puerto Rico and Parkes Observatory in Australia were designed to detect radio waves.
Infrared telescopes need to have their detectors cooled by liquid nitrogen to be able to gather useful signals. Since infrared radiation is predominantly
from thermal agitation, if the detectors were not cooled, the vibrations of the molecules in the antenna would be stronger than the signal being
collected.
The most famous of these infrared sensitive telescopes is the James Clerk Maxwell Telescope in Hawaii. The earliest telescopes, developed in the
seventeenth century, were optical telescopes, collecting visible light. Telescopes in the ultraviolet, X-ray, and γ -ray regions are placed outside the
atmosphere on satellites orbiting the Earth.
The Hubble Space Telescope (launched in 1990) gathers ultraviolet radiation as well as visible light. In the X-ray region, there is the Chandra X-ray
Observatory (launched in 1999), and in the γ -ray region, there is the new Fermi Gamma-ray Space Telescope (launched in 2008—taking the place
of the Compton Gamma Ray Observatory, 1991–2000.).
PhET Explorations: Color Vision
Make a whole rainbow by mixing red, green, and blue light. Change the wavelength of a monochromatic beam or filter white light. View the light
as a solid beam, or see the individual photons.
Figure 24.22 Color Vision (http://cnx.org/content/m42444/1.4/color-vision_en.jar)
878 CHAPTER 24 | ELECTROMAGNETIC WAVES
24.4 Energy in Electromagnetic Waves
Anyone who has used a microwave oven knows there is energy in electromagnetic waves. Sometimes this energy is obvious, such as in the
warmth of the summer sun. Other times it is subtle, such as the unfelt energy of gamma rays, which can destroy living cells.
Electromagnetic waves can bring energy into a system by virtue of their electric and magnetic fields. These fields can exert forces and move
charges in the system and, thus, do work on them. If the frequency of the electromagnetic wave is the same as the natural frequencies of the system
(such as microwaves at the resonant frequency of water molecules), the transfer of energy is much more efficient.
Connections: Waves and Particles
The behavior of electromagnetic radiation clearly exhibits wave characteristics. But we shall find in later modules that at high frequencies,
electromagnetic radiation also exhibits particle characteristics. These particle characteristics will be used to explain more of the properties of the
electromagnetic spectrum and to introduce the formal study of modern physics.
Another startling discovery of modern physics is that particles, such as electrons and protons, exhibit wave characteristics. This simultaneous











