densities are nearly equal. If there is a loss of fluid, the brain rests on the inside of the skull, causing severe headaches, constricted blood flow, and
serious damage. Spinal fluid pressure is measured by means of a needle inserted between vertebrae that transmits the pressure to a suitable
measuring device.
Bladder Pressure
This bodily pressure is one of which we are often aware. In fact, there is a relationship between our awareness of this pressure and a subsequent
increase in it. Bladder pressure climbs steadily from zero to about 25 mm Hg as the bladder fills to its normal capacity of 500 cm3 . This pressure
triggers the micturition reflex, which stimulates the feeling of needing to urinate. What is more, it also causes muscles around the bladder to
contract, raising the pressure to over 100 mm Hg, accentuating the sensation. Coughing, straining, tensing in cold weather, wearing tight clothes, and
experiencing simple nervous tension all can increase bladder pressure and trigger this reflex. So can the weight of a pregnant woman’s fetus,
especially if it is kicking vigorously or pushing down with its head! Bladder pressure can be measured by a catheter or by inserting a needle through
the bladder wall and transmitting the pressure to an appropriate measuring device. One hazard of high bladder pressure (sometimes created by an
obstruction), is that such pressure can force urine back into the kidneys, causing potentially severe damage.
Pressures in the Skeletal System
These pressures are the largest in the body, due both to the high values of initial force, and the small areas to which this force is applied, such as in
the joints.. For example, when a person lifts an object improperly, a force of 5000 N may be created between vertebrae in the spine, and this may be
applied to an area as small as 10 cm2 . The pressure created is P = F / A = (5000 N) / (10−3 m2 ) = 5.0×106 N/m2 or about 50 atm! This
pressure can damage both the spinal discs (the cartilage between vertebrae), as well as the bony vertebrae themselves. Even under normal
circumstances, forces between vertebrae in the spine are large enough to create pressures of several atmospheres. Most causes of excessive
pressure in the skeletal system can be avoided by lifting properly and avoiding extreme physical activity. (See Forces and Torques in Muscles and
Joints.)
There are many other interesting and medically significant pressures in the body. For example, pressure caused by various muscle actions drives
food and waste through the digestive system. Stomach pressure behaves much like bladder pressure and is tied to the sensation of hunger. Pressure
in the relaxed esophagus is normally negative because pressure in the chest cavity is normally negative. Positive pressure in the stomach may thus
force acid into the esophagus, causing “heartburn.” Pressure in the middle ear can result in significant force on the eardrum if it differs greatly from
atmospheric pressure, such as while scuba diving. The decrease in external pressure is also noticeable during plane flights (due to a decrease in the
weight of air above relative to that at the Earth’s surface). The Eustachian tubes connect the middle ear to the throat and allow us to equalize
pressure in the middle ear to avoid an imbalance of force on the eardrum.
Many pressures in the human body are associated with the flow of fluids. Fluid flow will be discussed in detail in the Fluid Dynamics and Its
Biological and Medical Applications.
Glossary
Archimedes’ principle: the buoyant force on an object equals the weight of the fluid it displaces
absolute pressure: the sum of gauge pressure and atmospheric pressure
adhesive forces: the attractive forces between molecules of different types
buoyant force: the net upward force on any object in any fluid
capillary action: the tendency of a fluid to be raised or lowered in a narrow tube
cohesive forces: the attractive forces between molecules of the same type
contact angle: the angle θ between the tangent to the liquid surface and the surface
density: the mass per unit volume of a substance or object
390 CHAPTER 11 | FLUID STATICS
diastolic pressure: the minimum blood pressure in the artery
diastolic pressure: minimum arterial blood pressure; indicator for the fluid balance
fluids: liquids and gases; a fluid is a state of matter that yields to shearing forces
gauge pressure: the pressure relative to atmospheric pressure
glaucoma: condition caused by the buildup of fluid pressure in the eye
intraocular pressure: fluid pressure in the eye
micturition reflex: stimulates the feeling of needing to urinate, triggered by bladder pressure
Pascal’s Principle: a change in pressure applied to an enclosed fluid is transmitted undiminished to all portions of the fluid and to the walls of its
container
pressure: the force per unit area perpendicular to the force, over which the force acts
pressure: the weight of the fluid divided by the area supporting it
specific gravity: the ratio of the density of an object to a fluid (usually water)
surface tension: the cohesive forces between molecules which cause the surface of a liquid to contract to the smallest possible surface area
systolic pressure: the maximum blood pressure in the artery
systolic pressure: maximum arterial blood pressure; indicator for the blood flow
Section Summary
11.1 What Is a Fluid?
• A fluid is a state of matter that yields to sideways or shearing forces. Liquids and gases are both fluids. Fluid statics is the physics of stationary
fluids.
11.2 Density
• Density is the mass per unit volume of a substance or object. In equation form, density is defined as
ρ = mV.
• The SI unit of density is kg/m3 .
11.3 Pressure
• Pressure is the force per unit perpendicular area over which the force is applied. In equation form, pressure is defined as
P = FA.
• The SI unit of pressure is pascal and 1 Pa = 1 N/m2 .
11.4 Variation of Pressure with Depth in a Fluid
• Pressure is the weight of the fluid mg divided by the area A supporting it (the area of the bottom of the container):
P = mg
A .
• Pressure due to the weight of a liquid is given by
P = hρg,
where P is the pressure, h is the height of the liquid, ρ is the density of the liquid, and g is the acceleration due to gravity.
11.5 Pascal’s Principle
• Pressure is force per unit area.
• A change in pressure applied to an enclosed fluid is transmitted undiminished to all portions of the fluid and to the walls of its container.
• A hydraulic system is an enclosed fluid system used to exert forces.
11.6 Gauge Pressure, Absolute Pressure, and Pressure Measurement
• Gauge pressure is the pressure relative to atmospheric pressure.
• Absolute pressure is the sum of gauge pressure and atmospheric pressure.
• Aneroid gauge measures pressure using a bellows-and-spring arrangement connected to the pointer of a calibrated scale.
• Open-tube manometers have U-shaped tubes and one end is always open. It is used to measure pressure.
• A mercury barometer is a device that measures atmospheric pressure.
11.7 Archimedes’ Principle
• Buoyant force is the net upward force on any object in any fluid. If the buoyant force is greater than the object’s weight, the object will rise to the
surface and float. If the buoyant force is less than the object’s weight, the object will sink. If the buoyant force equals the object’s weight, the
object will remain suspended at that depth. The buoyant force is always present whether the object floats, sinks, or is suspended in a fluid.
CHAPTER 11 | FLUID STATICS 391
• Archimedes’ principle states that the buoyant force on an object equals the weight of the fluid it displaces.
• Specific gravity is the ratio of the density of an object to a fluid (usually water).
11.8 Cohesion and Adhesion in Liquids: Surface Tension and Capillary Action
• Attractive forces between molecules of the same type are called cohesive forces.
• Attractive forces between molecules of different types are called adhesive forces.
• Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called
surface tension.
• Capillary action is the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary tube which is due to the relative strength of
cohesive and adhesive forces.
11.9 Pressures in the Body
• Measuring blood pressure is among the most common of all medical examinations.
• The pressures in various parts of the body can be measured and often provide valuable medical indicators.
• The shape of the eye is maintained by fluid pressure, called intraocular pressure.
• When the circulation of fluid in the eye is blocked, it can lead to a buildup in pressure, a condition called glaucoma.
• Some of the other pressures in the body are spinal and skull pressures, bladder pressure, pressures in the skeletal system.
Conceptual Questions
11.1 What Is a Fluid?
1. What physical characteristic distinguishes a fluid from a solid?
2. Which of the following substances are fluids at room temperature: air, mercury, water, glass?
3. Why are gases easier to compress than liquids and solids?
4. How do gases differ from liquids?
11.2 Density
5. Approximately how does the density of air vary with altitude?
6. Give an example in which density is used to identify the substance composing an object. Would information in addition to average density be
needed to identify the substances in an object composed of more than one material?
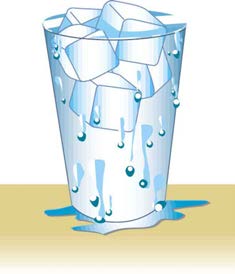
7. Figure 11.40 shows a glass of ice water filled to the brim. Will the water overflow when the ice melts? Explain your answer.
Figure 11.40
11.3 Pressure
8. How is pressure related to the sharpness of a knife and its ability to cut?
9. Why does a dull hypodermic needle hurt more than a sharp one?
10. The outward force on one end of an air tank was calculated in Example 11.2. How is this force balanced? (The tank does not accelerate, so the force must be balanced.)
11. Why is force exerted by static fluids always perpendicular to a surface?
12. In a remote location near the North Pole, an iceberg floats in a lake. Next to the lake (assume it is not frozen) sits a comparably sized glacier
sitting on land. If both chunks of ice should melt due to rising global temperatures (and the melted ice all goes into the lake), which ice chunk would
give the greatest increase in the level of the lake water, if any?
13. How do jogging on soft ground and wearing padded shoes reduce the pressures to which the feet and legs are subjected?
14. Toe dancing (as in ballet) is much harder on toes than normal dancing or walking. Explain in terms of pressure.
15. How do you convert pressure units like millimeters of mercury, centimeters of water, and inches of mercury into units like newtons per meter
squared without resorting to a table of pressure conversion factors?
11.4 Variation of Pressure with Depth in a Fluid
16. Atmospheric pressure exerts a large force (equal to the weight of the atmosphere above your body—about 10 tons) on the top of your body when
you are lying on the beach sunbathing. Why are you able to get up?
17. Why does atmospheric pressure decrease more rapidly than linearly with altitude?
392 CHAPTER 11 | FLUID STATICS
18. What are two reasons why mercury rather than water is used in barometers?
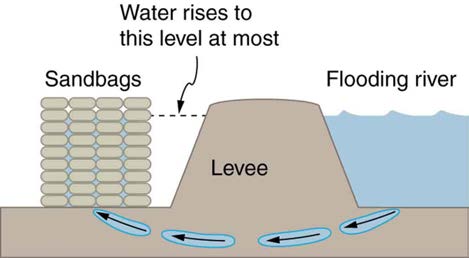
19. Figure 11.41 shows how sandbags placed around a leak outside a river levee can effectively stop the flow of water under the levee. Explain how
the small amount of water inside the column formed by the sandbags is able to balance the much larger body of water behind the levee.
Figure 11.41 Because the river level is very high, it has started to leak under the levee. Sandbags are placed around the leak, and the water held by them rises until it is the same level as the river, at which point the water there stops rising.
20. Why is it difficult to swim under water in the Great Salt Lake?
21. Is there a net force on a dam due to atmospheric pressure? Explain your answer.
22. Does atmospheric pressure add to the gas pressure in a rigid tank? In a toy balloon? When, in general, does atmospheric pressure not affect the
total pressure in a fluid?
23. You can break a strong wine bottle by pounding a cork into it with your fist, but the cork must press directly against the liquid filling the
bottle—there can be no air between the cork and liquid. Explain why the bottle breaks, and why it will not if there is air between the cork and liquid.
11.5 Pascal’s Principle
24. Suppose the master cylinder in a hydraulic system is at a greater height than the slave cylinder. Explain how this will affect the force produced at
the slave cylinder.
11.6 Gauge Pressure, Absolute Pressure, and Pressure Measurement
25. Explain why the fluid reaches equal levels on either side of a manometer if both sides are open to the atmosphere, even if the tubes are of
different diameters.
26. Figure 11.17 shows how a common measurement of arterial blood pressure is made. Is there any effect on the measured pressure if the
manometer is lowered? What is the effect of raising the arm above the shoulder? What is the effect of placing the cuff on the upper leg with the
person standing? Explain your answers in terms of pressure created by the weight of a fluid.
27. Considering the magnitude of typical arterial blood pressures, why are mercury rather than water manometers used for these measurements?
11.7 Archimedes’ Principle
28. More force is required to pull the plug in a full bathtub than when it is empty. Does this contradict Archimedes’ principle? Explain your answer.
29. Do fluids exert buoyant forces in a “weightless” environment, such as in the space shuttle? Explain your answer.
30. Will the same ship float higher in salt water than in freshwater? Explain your answer.
31. Marbles dropped into a partially filled bathtub sink to the bottom. Part of their weight is supported by buoyant force, yet the downward force on the
bottom of the tub increases by exactly the weight of the marbles. Explain why.
11.8 Cohesion and Adhesion in Liquids: Surface Tension and Capillary Action
32. The density of oil is less than that of water, yet a loaded oil tanker sits lower in the water than an empty one. Why?
33. Is surface tension due to cohesive or adhesive forces, or both?
34. Is capillary action due to cohesive or adhesive forces, or both?
35. Birds such as ducks, geese, and swans have greater densities than water, yet they are able to sit on its surface. Explain this ability, noting that
water does not wet their feathers and that they cannot sit on soapy water.
36. Water beads up on an oily sunbather, but not on her neighbor, whose skin is not oiled. Explain in terms of cohesive and adhesive forces.
37. Could capillary action be used to move fluids in a “weightless” environment, such as in an orbiting space probe?
38. What effect does capillary action have on the reading of a manometer with uniform diameter? Explain your answer.
39. Pressure between the inside chest wall and the outside of the lungs normally remains negative. Explain how pressure inside the lungs can
become positive (to cause exhalation) without muscle action.
CHAPTER 11 | FLUID STATICS 393
Problems & Exercises
14. What depth of mercury creates a pressure of 1.00 atm?
15. The greatest ocean depths on the Earth are found in the Marianas
11.2 Density
Trench near the Philippines. Calculate the pressure due to the ocean at
the bottom of this trench, given its depth is 11.0 km and assuming the
1. Gold is sold by the troy ounce (31.103 g). What is the volume of 1 troy
density of seawater is constant all the way down.
ounce of pure gold?
2. Mercury is commonly supplied in flasks containing 34.5 kg (about 76
16. Verify that the SI unit of hρg is N/m2 .
lb). What is the volume in liters of this much mercury?
17. Water towers store water above the level of consumers for times of
3. (a) What is the mass of a deep breath of air having a volume of 2.00
heavy use, eliminating the need for high-speed pumps. How high above
L? (b) Discuss the effect taking such a breath has on your body’s volume
a user must the water level be to create a gauge pressure of
and density.
3.00×105 N/m2 ?
4. A straightforward method of finding the density of an object is to
measure its mass and then measure its volume by submerging it in a
18. The aqueous humor in a person’s eye is exerting a force of 0.300 N
graduated cylinder. What is the density of a 240-g rock that displaces
on the 1.10-cm2 area of the cornea. (a) What pressure is this in mm
89.0 cm3 of water? (Note that the accuracy and practical applications
Hg? (b) Is this value within the normal range for pressures in the eye?
of this technique are more limited than a variety of others that are based
19. How much force is exerted on one side of an 8.50 cm by 11.0 cm
on Archimedes’ principle.)
sheet of paper by the atmosphere? How can the paper withstand such a
5. Suppose you have a coffee mug with a circular cross section and
force?
vertical sides (uniform radius). What is its inside radius if it holds 375 g of
20. What pressure is exerted on the bottom of a 0.500-m-wide by
coffee when filled to a depth of 7.50 cm? Assume coffee has the same
0.900-m-long gas tank that can hold 50.0 kg of gasoline by the weight of
density as water.
the gasoline in it when it is full?
6. (a) A rectangular gasoline tank can hold 50.0 kg of gasoline when full.
21. Calculate the average pressure exerted on the palm of a shot-putter’s
What is the depth of the tank if it is 0.500-m wide by 0.900-m long? (b)
hand by the shot if the area of contact is 50.0 cm2 and he exerts a
Discuss whether this gas tank has a reasonable volume for a passenger
car.
force of 800 N on it. Express the pressure in N/m2 and compare it with
7. A trash compactor can reduce the volume of its contents to 0.350 their
the 1.00×106 Pa pressures sometimes encountered in the skeletal
original value. Neglecting the mass of air expelled, by what factor is the
system.
density of the rubbish increased?
22. The left side of the heart creates a pressure of 120 mm Hg by
8. A 2.50-kg steel gasoline can holds 20.0 L of gasoline when full. What
exerting a force directly on the blood over an effective area of
is the average density of the full gas can, taking into account the volume
occupied by steel as well as by gasoline?
15.0 cm2. What force does it exert to accomplish this?
9. What is the density of 18.0-karat gold that is a mixture of 18 parts gold,
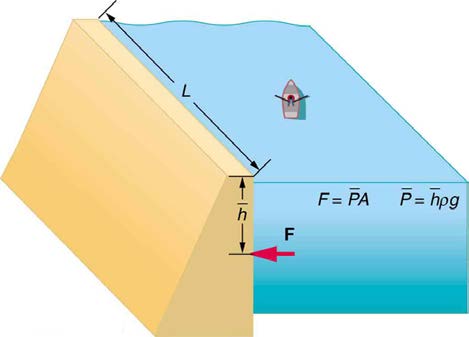
23. Show that the total force on a rectangular dam due to the water
5 parts silver, and 1 part copper? (These values are parts by mass, not
behind it increases with the square of the water depth. In particular, show
volume.) Assume that this is a simple mixture having an average density
that this force is given by F = ρgh 2 L / 2 , where ρ is the density of
equal to the weighted densities of its constituents.
10. There is relatively little empty space between atoms in solids and
water, h is its depth at the dam, and L is the length of the dam. You
liquids, so that the average density of an atom is about the same as
may assume the face of the dam is vertical. (Hint: Calculate the average
pressure exerted and multiply this by the area in contact with the water.
matter on a macroscopic scale—approximately 103 kg/m3 . The
(See Figure 11.42.)
nucleus of an atom has a radius about 10−5 that of the atom and
contains nearly all the mass of the entire atom. (a) What is the
approximate density of a nucleus? (b) One remnant of a supernova,
called a neutron star, can have the density of a nucleus. What would be
the radius of a neutron star with a mass 10 times that of our Sun (the
radius of the Sun is 7×108 m )?
11.3 Pressure
11. As a woman walks, her entire weight is momentarily placed on one
heel of her high-heeled shoes. Calculate the pressure exerted on the
floor by the heel if it has an area of 1.50 cm2 and the woman’s mass is
55.0 kg. Express the pressure in Pa. (In the early days of commercial
flight, women were not allowed to wear high-heeled shoes because
aircraft floors were too thin to withstand such large pressures.)
12. The pressure exerted by a phonograph needle on a record is
Figure 11.42
surprisingly large. If the equivalent of 1.00 g is supported by a needle, the 11.5 Pascal’s Principle
tip of which is a circle 0.200 mm in radius, what pressure is exerted on
the record in N/m2 ?
24. How much pressure is transmitted in the hydraulic system considered
in Example 11.6? Express your answer in pascals and in atmospheres.
13. Nail tips exert tremendous pressures when they are hit by hammers
25. What force must be exerted on the master cylinder of a hydraulic lift
because they exert a large force over a small area. What force must be
to support the weight of a 2000-kg car (a large car) resting on the slave
exerted on a nail with a circular tip of 1.00 mm diameter to create a
cylinder? The master cylinder has a 2.00-cm diameter and the sla






