4.0-1.7---Ionic bond-50-100% ionic
1.7-0.3---Polar Covalent bond-5-50% ionic
0.3-0.0---Non-Polar Covalent-0-5% ionic
Determine the type of bonding in the following compounds: KCl, CO, CaBr2, SiH4, MgS.
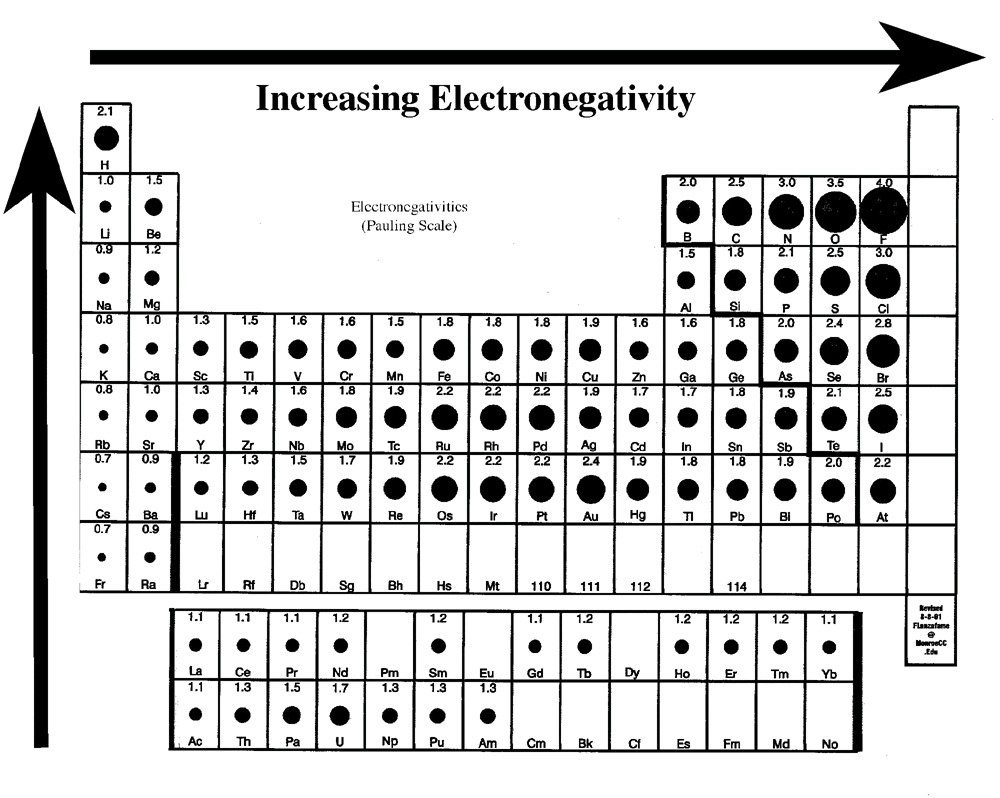
Figure 7.2.
Part II. Weak and strong electrolytes
Chemicals
tap water
0.1 M hydrochloric acid, HCl
0.1 M acetic acid, HC2 H 3 O 2
0.1 M sulfuric acid, H 2SO4
0.1 M sodium hydroxide, NaOH
0.1 M ammonia, NH3
0.1 M sodium acetate, NaC2 H 3 O 2
0.1 M sodium chloride, NaCl
0.1 M ammonium acetate, NH4 C 2 H 3 O 2
0.1 M ammonium chloride, NH4Cl
methanol, CH3OH
ethanol, C 2 H 5OH
sucrose solution, C 12 H 22 O 11
In today’s lab, you will be using a MicroLab conductivity probe to determine how well electrons flow through a given solution. First, you will need to calibrate the probe with a non-electrolyte (distilled water) and a very strong electrolyte. To quantify how well a solution conducts, we will assign numerical values to the conductance probe. A non-conducting solution will have a
conductance value of 0, a poor conducting solution will have a reading of 0 to 1,000, and good
conductors will have readings of 3,000 up.
Instructions for MicroLab Conductivity Experiment
Open the MicroLab Program by clicking on the Shortcut to MicroLab.exe tab on the desktop.
On the “Choose an Experiment Type” Tab, enter a name for the experiment, and then double click
on the MicroLab Experiment icon
Click “Add Sensor”, Choose sensor = Conductivity Probe
Choose an input, click on the red box that corresponds to the port that your conductivity sensor is connected to. Choose 20,000 microseconds
“Choose a Sensor”, click radial button that says Conductivity Probe. Click next.
Click “Perform New Calibration”
Click “Add Calibration Point” place the conductivity probe in the non-conductive standard
solution, while swirling wait until the value is constant and then enter 0.0 into the “Actual Value”
box in MicroLab and hit “ok”.
Again, Click “Add Calibration Point” place the conductivity probe in the conducting standard
solution, while swirling wait until the value is constant and then enter 1020 into the “Actual
Value” box in MicroLab and hit “ok”. Repeat for 3860 as the Actual Value.
Under Curve Fit Choices , click on “First order (linear)” and then “Accept and Save this
Calibration”, when prompted to “Enter the units for this calibration”, leave as is and click ok, save as your name-experiment-date. Click finish.
In the sensor area, left click on the conductivity icon and drag it to the Y-axis over “data source two”, also click and drag to column B on the spreadsheet and also click and drag to the digital display window.
When ready to obtain data, click start.
This is very important: Be sure to thoroughly since the probe with DI water between every use.
Beginning with the tap water, measure the conductance of each of the following solutions. Using the information provided in the lab manual, classify each solution as a non-, weak, or strong
electrolyte. For those solutions that are electrolytes, record the ions present in solution.
Part III. Electrolyte strength and reaction rate
Chemicals
calcium carbonate powder - shake once
1 M HCl - stopper it
1 M HC2 H 3 O 2
0.5 M H 2SO4
Test tube gas collection apparatus - end at 20mL
Measure 2 g of powdered calcium carbonate ( CaCO3) onto a piece of weigh paper. Obtain 30 mL
of 1 M HCl in a graduated cylinder. Pour the acid into the test tube apparatus. Add the calcium carbonate to the acid and QUICKLY stopper the tube to begin collecting gas. Record the time it
takes to collect 20 mL of gas. The acid may react very fast with the CaCO3 generating the gas very rapidly. Clean out the test tube apparatus and repeat the experiment using 1 M HC2 H 3 O 2 and 0.5
M H 2SO4.
Part IV. Chemical reactions
Chemicals
0.01 M calcium hydroxide, Ca(OH)2
Plastic straws
Plastic straws
Obtain ~20 mL of saturated calcium hydroxide solution. Make sure it is clear and colorless. Place the conductivity probe in the solution and begin monitoring it conductivity. With your straw,
slowly exhale into the solution. Note any observations in the solution and the conductivity.
Pre-Lab 5: Bonding 07
(Total 10 Points)
Hopefully here for the Pre-Lab Name(Print then sign): ___________________________________________________
Lab Day: ___________________Section: ________TA__________________________
This assignment must be completed individually and turned in to your TA at the beginning of lab.
You will not be allowed to begin the lab until you have completed this assignment.
Part I. Bonding of chemicals in solution
1. Write out the formulas of the following acids:
phosphoric ____________________
perchloric ____________________
nitric ____________________
sulfuric __________________
hydrochloric ____________________
acetic ____________________
1. Write out the formulas of the following bases:
calcium hydroxide ____________________
potassium hydroxide ____________________
sodium hydroxide ____________________
ammonia ____________________
1. Write out the formulas of the following salts:
potassium chromate ____________________
potassium sulfate ____________________
copper(II) nitrate ____________________
calcium carbonate ____________________
potassium iodide ____________________
Report 5: Bonding 07
Hopefully here for the Report Form Note: In preparing this report you are free to use references and consult with others. However, you may not copy from other students’ work (including your laboratory partner) or misrepresent your own data (see honor code).
Name(Print then sign): ___________________________________________________
Lab Day: ___________________Section: ________TA__________________________
Part I. Predicting bond type through electronegativity differences.
Table 7.1.
Chemical Formula Electroneg (1) Electroneg (2) Diff Electroneg Type of bond
KCl
CO
CaBr 2
SiH 4
MgS
Part II. Weak and strong electrolytes
Table 7.2.
Solution Tested
Numerical Output Electrolyte Strength Ions Present
0.1 M HCl
0.1 M HC2 H 3 O 2
0.1 M H 2SO4
0.1 M NaOH
0.1 M NH3
0.1 M NaC2 H 3 O 2
0.1 M NaCl
0.1 M NH4 C 2 H 3 O 2
0.1 M NH4Cl
CH 3 OH
C 2 H 5 OH
Sucrose
Tap water
1. Why do we use deionized water instead of tap water when making solutions for conductivity
measurements?
Part III. Electrolyte strength and reaction rate
2. Time to collect 20 mL of gas using 1 M HCl _______________________. Write the reaction of
HCl with CaCO3.
3. Time to collect 20 mL of gas using 1 M HC2 H 3 O 2_______________________. Write the reaction of HC2 H 3 O 2 with CaCO3.
4. Time to collect 20 mL of gas using 0.5 M H 2SO4_________________________.Write the
reaction of H 2SO4 with CaCO3.
5. Why does it take different lengths of time to collect 20 mL of gas?
6. Based on the time it took to collect 20 mL of gas, rank the acids in the order of increasing strength.
7. Why did we use 0.5 M H 2SO4 instead of 1.0 M H 2SO4?
Part IV. Chemical reactions
8. Write the chemical reaction for calcium hydroxide with your exhaled breath.
9. Write your observations for the reaction that took place (i.e. appearance, conductivity, etc.) 10. When in separate solutions, aqueous ammonia, NH3(aq) and acetic acid HC2 H 3 O 2 conduct electricity equally well. However, when the two solutions are mixed a substantial increase in
electrical conductivity is observed. Explain.
11. Separately, ammonium sulfate and barium hydroxide solutions are very good conductors.
When the two solutions are mixed a substantial decrease in conductivity is observed. Rationalize this.
Solutions

Chapter 8. Solid State and Superconductors
Solid State Structures and Superconductors
Objectives
Build examples of: simple cubic, body centered cubic and face centered cubic cells.
Understand and familiarize with three-dimensionality of solid state structures.
Understand how binary ionic compounds (compounds made up of two different types of ions)
pack in a crystal lattice.
Observe the special electromagnetic characteristics of superconducting materials using 1,2,3-
superconductor
, discovered in 1986 by Dr. Paul Chu at the University of Houston.
Grading
Your grade will be determined according to the following
Pre-lab (10%)
Lab report form. (80%)
TA points (10%)
Before coming to lab:
Read introduction and model kits section
Complete prelab exercise
Introduction
From the three states of matter, the solid state is the one in which matter is highly condensed. In the solid state, when atoms, molecules or ions pack in a regular arrangement which can be
repeated "infinitely" in three dimensions, a crystal is formed. A crystalline solid, therefore, possesses long-range order; its atoms, molecules, or ions occupy regular positions which repeat in three dimensions. On the other hand an amorphous solid does not possess any long-range order.
Glass is an example of an amorphous solid. And even though amorphous solids have very


interesting properties in their own right that differ from those of crystalline materials, we will not consider their structures in this laboratory exercise.
The simplest example of a crystal is table salt, or as we chemists know it, sodium chloride (NaCl).
A crystal of sodium chloride is composed of sodium cations (
) and chlorine anions (
) that
are arranged in a specific order and extend in three dimensions. The ions pack in a way that
maximizes space and provides the right coordination for each atom (ion). Crystals are three
dimensional, and in theory, the perfect crystal would be infinite. Therefore instead of having a molecular formula, crystals have an empirical formula based on stoichiometry. Crystalline
structures are defined by a unit cell which is the smallest unit that contains the stoichiometry and the “spatial arrangement” of the whole crystal. Therefore a unit cell can be seen as the building block of a crystal.
The crystal lattice
In a crystal, the network of atoms, molecules, or ions is known as a crystal lattice or simply as a lattice. In reality, no crystal extends infinitely in three dimensions and the structure (and also properties) of the solid will vary at the surface (boundaries) of the crystal. However, the number of atoms located at the surface of a crystal is very small compared to the number of atoms in the interior of the crystal, and so, to a first approximation, we can ignore the variations at the surface for much of our discussion of crystals. Any location in a crystal lattice is known as a lattice point.
Since the crystal lattice repeats in three dimensions, there will be an entire set of lattice points which are identical. That means that if you were able to make yourself small enough and stand at any such lattice point in the crystal lattice, you would not be able to tell which lattice point of the set you were at – the environment of a lattice point is identical to each correspondent lattice point throughout the crystal. Of course, you could move to a different site (a non-correspondent lattice point) which would look different. This would constitute a different lattice point. For example, when we examine the sodium chloride lattice later, you will notice that the environment of each sodium ion is identical. If you were to stand at any sodium ion and look around, you would see the same thing. If you stood at a chloride ion, you would see a different environment but that
environment would be the same at every chloride ion. Thus, the sodium ion locations form one set of lattice points and the chloride ion locations form another set. However, lattice points not only exist in atom positions. We could easily define a set of lattice points at the midpoints between the sodium and chloride ions in the crystal lattice of sodium chloride.
The unit cell
Since the crystal lattice is made up of a regular arrangement which repeats in three dimensions, we can save ourselves a great deal of work by considering the simple repeating unit rather than the entire crystal lattice. The basic repeating unit is known as the unit cell. Crystalline solids often have flat, well-defined faces that make definite angles with their neighbors and break cleanly
when struck. These faces lie along well-defined directions in the unit cell.
The unit cell is the smallest, most symmetrical repeating unit that, when translated in three
dimensions, will generate the entire crystal lattice.
It is possible to have a number of different choices for the unit cell. By convention, the unit cell that reflects the highest symmetry of the lattice is the one that is chosen. A unit cell may be thought of as being like a brick which is used to build a building (a crystal). Many bricks are stacked together to create the entire structure. Because the unit cell must translate in three
dimensions, there are certain geometrical constraints placed upon its shape. The main criterion is that the opposite faces of the unit cell must be parallel. Because of this restriction there are only six parameters that we need to define in order to define the shape of the unit cell. These include three edge lengths a, b, and c and three angles α, β and γ. Once these are defined all other distances and angles in the unit cell are set. As a result of symmetry, some of these angles and edge lengths may be the same. There are only seven different shapes for unit cells possible. These are given in the chart below.
Table 8.1.
Highest Type of Symmetry Element
Unit Cell Type
Restrictions on Unit Cell Parameters
Required
a is not equal to b is not equal to c; α is
no symmetry is required, an
Triclinic
not equal to β is not equal to γ.
inversioncenter may be present
a is not equal to b is not equal to c α = highest symmetry element allowed
Monoclinic
γ 90 ° β is not equal to 90 °.
is aC2 axis or a mirror plane
has three mutually
a is not equal to b is not equal to c α =
Orthorhombic
perpendicularmirror planes and/or
β = γ 90 °
C2 axes
Tetragonal
a =b is not equal to c α = β = γ 90 °
has one C4 axis
Cubic
a =b =c α = β = γ 90 °
has C3 and C4 axes
Hexagonal,
a =b is not equal to c α = β = 90 °
C6 axis (hexagonal); C3 axis
Trigonal
γ 120 °
(trigonal)
Rhombohedral* a =b =c α = β = γ is not equal to 90 °
C3 axis (trigonal)
*There is some discussion about whether the rhombohedral unit cell is a different group or is
really a subset of the trigonal/hexagonal types of unit cell.
Stoichiometry
You will be asked to count the number of atoms in each unit cell in order to determine the
stoichiometry (atom-to-atom ratio) or empirical formula of the compound. However, it is
important to remember that solid state structures are extended, that is, they extend out in all directions such that the atoms that lie on the corners, faces, or edges of a unit cell will be shared with other unit cells, and therefore will only contribute a fraction of that boundary atom. As you build crystal lattices in these exercises you will note that eight unit cells come together at a corner. Thus, an atom which lies exactly at the corner of a unit cell will be shared by eight unit cells which means that only ⅛ of the atom contributes to the stoichiometry of any particular unit cell. Likewise, if an atom is on an edge, only ¼ of the atom will be in a unit cell because four unit cells share an edge. An atom on a face will only contribute ½ to each unit cell since the face is shared between two unit cells.
It is very important to understand that the stoichiometry of the atoms within the unit cell must reflect the composition of the bulk material.
Binding forces in a crystal
The forces which stabilize the crystal may be ionic (electrostatic) forces, covalent bonds, metallic bonds, van der Waals forces, hydrogen bonds, or combination of these. The properties of the
crystal will change depending upon what types of bonding is involved in holding the atoms,
molecules, or ions in the lattice. The fundamental types of crystals based upon the types of forces that hold them together are: metallic in which metal cations held together by a sea of electrons, ionic in which cations and anions held together by predominantly electrostatic attractions, and network in which atoms bonded together covalently throughout the solid (also known as covalent
crystal or covalent network).
Close-packing
Close-packing of spheres is one example of an arrangement of objects that forms an extended
structure. Extended close-packing of spheres results in 74% of the available space being occupied by spheres (or atoms), with the remainder attributed to the empty space between the spheres. This is the highest space-filling efficiency of any sphere-packing arrangement. The nature of extended structures as well as close-packing, which occurs in two forms called hexagonal close packing
(hcp) and cubic close packing (ccp), will be explored in this lab activity. Sixty-eight of the ninety naturally occurring elements are metallic elements. Forty of these metals have three-dimensional submicroscopic structures that can be described in terms of close-packing of spheres. Another
sixteen of the sixty-eight naturally occurring metallic elements can be described in terms of a different type of extended structure that is not as efficient at space-filling. This structure occupies only 68% of the available space in the unit cell. This second largest subgroup exhibits a sphere packing arrangement called body-centered cubic (bcc).
You should be able to calculate the % of void space using simple geometry.
Packing of more than one type of ion (binary compounds) in a crystal lattice
A very useful way to describe the extended structure of many substances, particularly ionic
compounds, is to assume that ions, which may be of different sizes, are spherical. The structure then is based on some type of sphere packing scheme exhibited by the larger ion, with the smaller ion occupying the unused space (interstitial sites). In structures of this type, coordination number refers to the number of nearest neighbors of opposite charge. Salts exhibiting these packing
arrangements will be explored in this lab activity.
Coordination number and interstitial sites
When spherical objects of equal size are packed in some type of arrangement, the number of
nearest neighbors to any given sphere is dependent upon the efficiency of space filling. The
number of nearest neighbors is called the coordination number and abbreviated as CN. The sphere packing schemes with the highest space-filling efficiency will have the highest CN. Coordination number will be explored in this lab activity. A useful way to describe extended structures, is by using the unit cell which as discussed above is the repeating three-dimensional pattern for


extended structures. A unit cell has a pattern for the objects as well as for the void spaces. The remaining unoccupied space in any sphere packing scheme is found as void space. This void space occurs between the spheres and gives rise to so-called interstitial sites.
Synthesis of solid state materials
There exist many synthetic methods to make crystalline solids. Traditional solid state chemical reactions are often slow and require high temperatures and long periods of time for reactants to form the desire compound. They also require that reactants are mixed in the solid state by
grinding two solids together. In this manner the mixture formed is heterogeneous (i.e. not in the same phase), and high temperatures are required to increase the mobility of the ions that are being formed into the new solid binary phase. Another approach to get solid state binary structures is using a precursor material such as a metal carbonate, that upon decomposition at high
temperatures loses gaseous
resulting in very fine particles of the corresponding metal oxide
(e.g.,
).
X-ray crystallography
To determine the atomic or molecular structure of a crystal diffraction of X-rays is used. It was observed that visible light can be diffracted by the use of optical grids, because these are arranged in a regular manner. Energy sources such as X-rays have such small wavelengths that only “grids”
the size of atoms will be able to diffract X-rays. As mentioned before a crystal has regular
molecular array, and therefore it is possible, to use X-ray diffraction to determine the location of the atoms in crystal lattice. When such an experiment is carried out we say that we have
determined the crystal structure of the substance. The study of crystal structures is known as
crystallography and it is one of the most powerful techniques used today to characterize new
compounds. You will discuss the principles behind X-ray diffraction in the lecture part of this course.
Superconductors
A superconductor is an element, or compound that will conduct electricity without resistance
when it is below a certain temperature. Without resistance the electrical current will flow
continuously in a closed loop as long as the material is kept below an specific temperature. Since the electrical resistance is zero, supercurrents are generated in the material to exclude the
magnetic fields from a magnet brought near it. The currents which cancel the external field
produce magnetic poles opposite to the poles of the permanent magnet, repelling them to provide









