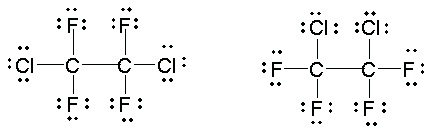Figure 5.18.
We conclude that an oxygen atom can satisfy its valence of 2 by forming two single bonds or by
forming one double bond. In both cases, we can understand the stability of the resulting molecules
by in terms of an octet of valence electrons.
Interpretation of Lewis Structures
Before further developing our model of chemical bonding based on Lewis structures, we pause to
consider the interpretation and importance of these structures. It is worth recalling that we have
developed our model based on observations of the numbers of bonds formed by individual atoms
and the number of valence electrons in each atom. In general, these structures are useful for
predicting whether a molecule is expected to be stable under normal conditions. If we cannot draw
a Lewis structure in which each carbon, oxygen, nitrogen, or halogen has an octet of valence
electrons, then the corresponding molecule probably is not stable. Consideration of bond strengths
and bond lengths enhances the model by revealing the presence of double and triple bonds in the
Lewis structures of some molecules.
At this point, however, we have observed no information regarding the geometries of molecules.
For example, we have not considered the angles measured between bonds in molecules.
Consequently, the Lewis structure model of chemical bonding does not at this level predict or
interpret these bond angles. (This will be considered here. ) Therefore, although the Lewis structure of methane is drawn as shown here.
Figure 5.19.

This does not imply that methane is a flat molecule, or that the angles between CH bonds in methane is 90°. Rather, the structure simply reveals that the carbon atom has a complete octet of
valence electrons in a methane molecule, that all bonds are single bonds, and that there are no
non-bonding electrons. Similarly, one can write the Lewis structure for a water molecule in two
apparently different ways, shown here.
Figure 5.20.
However, it is very important to realize that these two structures are identical in the Lewis model, because both show that the oxygen atom has a complete octet of valence electrons, forms two
single bonds with hydrogen atoms, and has two pairs of unshared electrons in its valence shell. In
the same way, the two structures for Freon 114 shown here are also identical.
Figure 5.21.
These two drawings do not represent different structures or arrangements of the atoms in the
bonds.
Finally, we must keep in mind that we have drawn Lewis structures strictly as a convenient tool
for our understanding of chemical bonding and molecular stability. It is based on commonly
observed trends in valence, bonding, and bond strengths. These structures must not be mistaken as
observations themselves, however. As we encounter additional experimental observations, we
must be prepared to adapt our Lewis structure model to fit these observations, but we must never
adapt our observations to fit the Lewis model.
Extensions of the Lewis Structure Model
With these thoughts in mind, we turn to a set of molecules which challenge the limits of the Lewis
model in describing molecular structures. First, we note that there are a variety of molecules for
which atoms clearly must bond in such a way as to have more than eight valence electrons. A
conspicuous example is SF 6, where the sulfur atom is bonded to six F atoms. As such, the S atom must have 12 valence shell electrons to form 6 covalent bonds. Similarly, the phosphorous atom in
P Cl5 has 10 valence electrons in 5 covalent bonds, the Cl atom in Cl F 3 has 10 valence electrons in 3 covalent bonds and two lone pairs. We also observe the interesting compounds of the noble gas
atoms, e.g. Xe O 3, where noble gas atom begins with eight valence electrons even before forming any bonds. In each of these cases, we note that the valence of the atoms S, P, Cl, and Xe are
normally 2, 3, 1, and 0, yet more bonds than this are formed. In such cases, it is not possible to
draw Lewis structures in which S, P, Cl, and Xe obey the octet rule. We refer to these molecules as
"expanded valence" molecules, meaning that the valence of the central atom has expanded beyond the expected octet.
There are also a variety of molecules for which there are too few electrons to provide an octet for
every atom. Most notably, Boron and Aluminum, from Group III, display bonding behavior
somewhat different than we have seen and thus less predictable from the model we have
developed so far. These atoms have three valence shell electrons, so we might predict a valence of
5 on the basis of the octet rule. However, compounds in which boron or aluminum atoms form
five bonds are never observed, so we must conclude that simple predictions based on the octet rule
are not reliable for Group III.
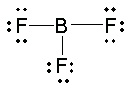
Consider first boron trifluoride, BF 3. The bonding here is relatively simple to model with a Lewis structure if we allow each valence shell electron in the boron atom to be shared in a covalent bond
with each fluorine atom.
Figure 5.22.
Note that, in this structure, the boron atom has only six valence shell electrons, but the octet rule
is obeyed by the fluorine atoms.
We might conclude from this one example that boron atoms obey a sextet rule. However, boron
will form a stable ion with hydrogen, BH –
4 , in which the boron atom does have a complete octet.
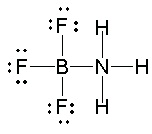
In addition, BF 3 will react with ammonia NH 3 for form a stable compound, NH 3 BF 3, for which a Lewis structure can be drawn in which boron has a complete octet, shown here.
Figure 5.23.
Compounds of aluminum follow similar trends. Aluminum trichloride, AlCl3, aluminum hydride,
Al H 3, and aluminum hydroxide, Al( OH)3, all indicate a valence of 3 for aluminum, with six valence electrons in the bonded molecule. However, the stability of aluminum hydride ions,
Al H –
4 , indicates that Al can also support an octet of valence shell electrons as well.
We conclude that, although the octet rule can still be of some utility in understanding the
chemistry of Boron and Aluminum, the compounds of these elements are less predictable from the
octet rule. This should not be disconcerting, however. The octet rule was developed in The Section
Called “Observation 1: Valence And The Periodic Table” on the basis of the observation that, for
elements in Groups IV through VIII, the number of valence electrons plus the most common
valence is equal to eight. Elements in Groups I, II, and III do not follow this observation most
commonly.
Resonance Structures
Another interesting challenge for the Lewis model we have developed is the set of molecules for
which it is possible to draw more than one structure in agreement with the octet rule. A notable
example is the nitric acid molecule, HNO 3, where all three oxygens are bonded to the nitrogen.
Two structures can be drawn for nitric acid with nitrogen and all three oxygens obeying the octet
rule.
In each structure, of the oxygens not bonded to hydrogen, one shares a single bond with nitrogen
while the other shares a double bond with nitrogen. These two structures are not identical, unlike
the two freon structures in Figure 5.12, because the atoms are bonded differently in the two structures.
Review and Discussion Questions
Exercise 1.
Compounds with formulae of the form CnH 2 n+2 are often referred to as "saturated" hydrocarbons.
Using Lewis structures, explain how and in what sense these molecules are "saturated."
Exercise 2.
Molecules with formulae of the form CnH 2 n+1 ( e.g. CH 3, C 2 H 5) are called "radicals" and are extremely reactive. Using Lewis structures, explain the reactivity of these molecules.
Exercise 3.
State and explain the experimental evidence and reasoning which shows that multiple bonds are
stronger and shorter than single bonds.
Exercise 4.
Compare N 2 to H 4 N 2. Predict which bond is stronger and explain why.
Exercise 5.
Explain why the two Lewis structures for Freon 114, shown in Figure 21Figure 5.21, are identical.
Draw a Lewis structures for an isomer of Freon 114, that is, another molecule with the same
molecular formula as Freon 114 but a different structural formula.
Solutions
Chapter 6. Molecular Geometry and Electron Domain
Theory
Foundation
We begin by assuming a Lewis structure model for chemical bonding based on valence shell
electron pair sharing and the octet rule. We thus assume the nuclear structure of the atom, and we
further assume the existence of a valence shell of electrons in each atom which dominates the
chemical behavior of that atom. A covalent chemical bond is formed when the two bonded atoms
share a pair of valence shell electrons between them. In general, atoms of Groups IV through VII
bond so as to complete an octet of valence shell electrons. A number of atoms, including C, N, O,
P, and S, can form double or triple bonds as needed to complete an octet. We know that double
bonds are generally stronger and have shorter lengths than single bonds, and triple bonds are
stronger and shorter than double bonds.
Goals
We should expect that the properties of molecules, and correspondingly the substances which they
comprise, should depend on the details of the structure and bonding in these molecules. The
relationship between bonding, structure, and properties is comparatively simple in diatomic
molecules, which contain two atoms only, e.g. HCl or O 2. A polyatomic molecule contains more than two atoms. An example of the complexities which arise with polyatomic molecules is
molecular geometry: how are the atoms in the molecule arranged with respect to one another? In a
diatomic molecule, only a single molecular geometry is possible since the two atoms must lie on a
line. However, with a triatomic molecule (three atoms), there are two possible geometries: the
atoms may lie on a line, producing a linear molecule, or not, producing a bent molecule. In
molecules with more than three atoms, there are many more possible geometries. What
geometries are actually observed? What determines which geometry will be observed in a
particular molecule? We seek a model which allows us to understand the observed geometries of
molecules and thus to predict these geometries.
Once we have developed an understanding of the relationship between molecular structure and
chemical bonding, we can attempt an understanding of the relationship of he structure and
bonding in a polyatomic molecule to the physical and chemical properties we observe for those
molecules.
Observation 1: Geometries of molecules
The geometry of a molecule includes a description of the arrangements of the atoms in the
molecule. At a simple level, the molecular structure tells us which atoms are bonded to which. At
a more detailed level, the geometry includes the lengths of all of these bonds, that is, the distances
between the atoms which are bonded together, and the angles between pairs of bonds. For
example, we find that in water, H 2 O, the two hydrogens are bonded to the oxygen and each O-H
bond length is 95.72pm (where 1pm=10-12 m). Furthermore, H 2 O is a bent molecule, with the H-O-H angle equal to 104.5°. (The measurement of these geometric properties is difficult, involving
the measurement of the frequencies at which the molecule rotates in the gas phase. In molecules
in crystalline form, the geometry of the molecule is revealed by irradiating the crystal with x-rays
and analyzing the patterns formed as the x-rays diffract off of the crystal.)
Not all triatomic molecules are bent, however. As a common example, CO 2 is a linear molecule.
Larger polyatomics can have a variety of shapes, as illustrated in Figure 6.1. Ammonia, NH 3, is a pyramid-shaped molecule, with the hydrogens in an equilateral triangle, the nitrogen above the
plane of this triangle, and a H-N-H angle equal to 107°. The geometry of CH 4 is that of a
tetrahedron, with all H-C-H angles equal to 109.5°. (See also Figure 6.2a.) Ethane, C 2 H 6, has a geometry related to that of methane. The two carbons are bonded together, and each is bonded to
three hydrogens. Each H-C-H angle is 109.5° and each H-C-C angle is 109.5°. By contrast, in
ethene, C 2 H 4, each H-C-H bond angle is 116.6° and each H-C-C bond angle is 121.7°. All six atoms of ethene lie in the same plane. Thus, ethene and ethane have very different geometries,
despite the similarities in their molecular formulae.
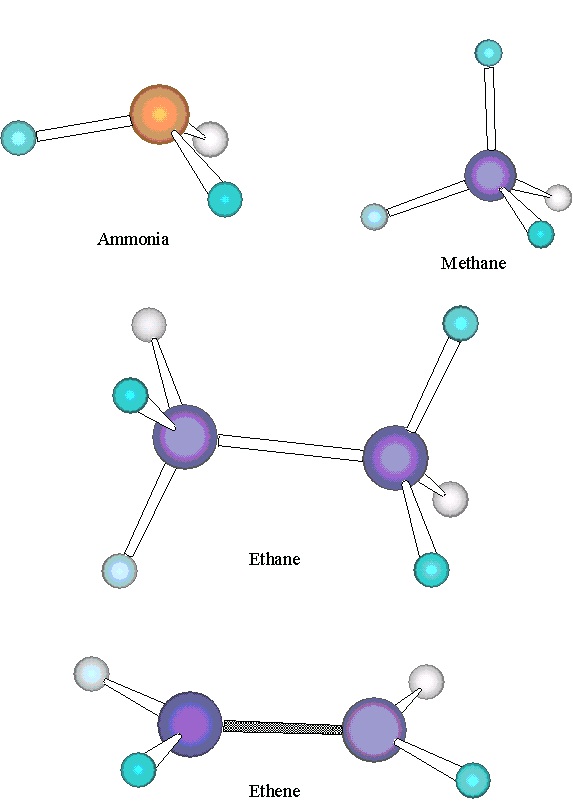
Figure 6.1. Molecular Structures
We begin our analysis of these geometries by noting that, in the molecules listed above which do
not contain double or triple bonds ( H 2 O, NH 3, CH 4and C 2 H 6), the bond angles are very similar, each equal to or very close to the tetrahedral angle 109.5°. To account for the observed angle, we
begin with our valence shell electron pair sharing model, and we note that, in the Lewis structures
of these molecules, the central atom in each bond angle of these molecules contains four pairs of
valence shell electrons. For methane and ethane, these four electron pairs are all shared with
adjacent bonded atoms, whereas in
ammonia or water, one or two (respectively) of the electron pairs are not shared with any other
atom. These unshared electron pairs are called lone pairs . Notice that, in the two molecules with no lone pairs, all bond angles are exactly equal to the tetrahedral angle, whereas the bond angles are only close in the molecules with lone pairs
One way to understand this result is based on the mutual repulsion of the negative charges on the
valence shell electrons. Although the two electrons in each bonding pair must remain relatively
close together in order to form the bond, different pairs of electrons should arrange themselves in
such a way that the distances between the pairs are as large as possible. Focusing for the moment
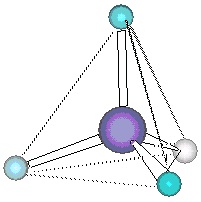
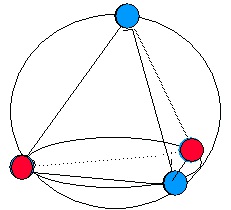
on methane, the four pairs of electrons must be equivalent to one another, since the four C-H
bonds are equivalent, so we can assume that the electron pairs are all the same distance from the
central carbon atom. How can we position four electron pairs at a fixed distance from the central
atom but as far apart from one another as possible? A little reflection reveals that this question is
equivalent to asking how to place four points on the surface of a sphere spread out from each other
as far apart as possible. A bit of experimentation reveals that these four points must sit at the
corners of a tetrahedron, an equilateral triangular pyramid, as may be seen in Figure 6.2b. If the carbon atom is at the center of this tetrahedron and the four electron pairs at placed at the corners,
then the hydrogen atoms also form a tetrahedron about the carbon. This is, as illustrated in
Figure 6.2a, the correct geometry of a methane molecule. The angle formed by any two corners of a tetrahedron and the central atom is 109.5°, exactly in agreement with the observed angle in
methane. This model also works well in predicting the bond angles in ethane.
(a) The dotted lines illustrate that the hydrogens form a tetrahedron about the carbon atom.
(b) The same tetrahedron is formed by placing four points on a sphere as far apart from one another as possible.
Figure 6.2. Tetrahedral Structure of Methane
We conclude that molecular geometry is determined by minimizing the mutual repulsion of the
valence shell electron pairs. As such, this model of molecular geometry is often referred to as the
valence shell electron pair repulsion (VSEPR) theory . For reasons that will become clear,
extension of this model implies that a better name is the Electron Domain (ED) Theory .
This model also accounts, at least approximately, for the bond angles of H 2 O and NH 3. These molecules are clearly not tetrahedral, like CH 4, since neither contains the requisite five atoms to form the tetrahedron. However, each molecule does contain a central atom surrounded by four
pairs of valence shell electrons. We expect from our Electron Domain model that those four pairs
should be arrayed in a tetrahedron, without regard to whether they are bonding or lone-pair
electrons. Then attaching the hydrogens (two for oxygen, three for nitrogen) produces a prediction
of bond angles of 109.5°, very close indeed to the observed angles of 104.5° in H 2 O and 107° in NH 3.
Note, however, that we do not describe the geometries of H 2 O and NH 3 as "tetrahedral," since the atoms of the molecules do not form tetrahedrons, even if the valence shell electron pairs do. (It is worth noting that these angles are not exactly equal to 109.5°, as in methane. These deviations will
be discussed later. )
We have developed the Electron Domain model to this point only for geometries of molecules
with four pairs of valence shell electrons. However, there are a great variety of molecules in which
atoms from Period 3 and beyond can have more than an octet of valence electrons. We consider
two such molecules illustrated in Figure 6.3.
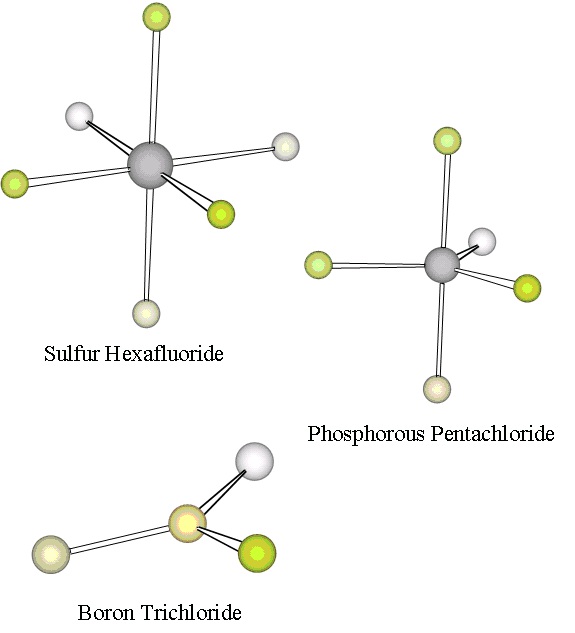
Figure 6.3. More Molecular Structures
First, P Cl5 is a stable gaseous compound in which the five chlorine atoms are each bonded to the phosphorous atom. Experiments reveal that the geometry of P Cl5 is that of a trigonal bipyramid : three of the chlorine atoms form an equilateral triangle with the P atom in the center, and the other
two chlorine atoms are on top of and below the P atom. Thus there must be 10 valence shell
electrons around the phosphorous atom. Hence, phosphorous exhibits what is called an expanded
valence in P Cl5. Applying our Electron Domain model, we expect the five valence shell electron pairs to spread out optimally to minimize their repulsions. The required geometry can again be
found by trying to place five points on the surface of a sphere with maximum distances amongst
these points. A little experimentation reveals that this can be achieved by placing the five points
to form a trigonal bipyramid. Hence, Electron Domain theory accounts for the geometry of P Cl5.
Second, SF 6 is a fairly unreactive gaseous compound in which all six fluorine atoms are bonded to the central sulfur atom. Again, it is clear that the octet rule is violated by the sulfur atom, which
must therefore have an expanded valence. The observed geometry of SF 6, as shown in Figure 6.3,
is highly symmetric: all bond lengths are identical and all bond angles are 90°. The F atoms form
an octahedron about the central S atom: four of the F atoms form a square with the S atom at the
center, and the other two F atoms are above and below the S atom. To apply our Electron Domain
model to understand this geometry, we must place six points, representing the six electron pairs
about the central S atom, on the surface of a sphere with maximum distances between the points.
The requisite geometry is found, in fact, to be that of an octahedron, in agreement with the
observed geometry.
As an example of a molecule with an atom with less than an octet of valence shell electrons, we
consider boron trichloride, B Cl3. The geometry of B Cl3 is also given in Figure 6.3: it is trigonal planar , with all four atoms lying in the same plane, and all Cl-B-Cl bond angles equal to 120°.
The three Cl atoms form an equilateral triangle. The Boron atom has only three pairs of valence
shell electrons in B Cl3. In applying Electron Domain theory to understand this geometry, we must place three points on the surface of a sphere with maximum distance between points. We find that
the three points form an equilateral triangle in a plane with the center of the sphere, so Electron
Domain is again in accord with the observed geometry.
We conclude from these predictions and observations that the Electron Domain model is a
reasonably accurate way to understand molecular geometries, even in molecules which violate the
octet rule.
Observation 2: Molecules with Double or Triple Bonds
In each of the molecules considered up to this point, the electron pairs are either in single bonds or
in lone pairs. In current form, the Electron Domain model does not account for the observed
geometry of C 2 H 4, in which each H-C-H bond angle is 116.6° and each H-C-C bond angle is 121.7° and all six atoms lie in the same plane. Each carbon atom in this molecule is surrounded by
four pairs of electrons, all of which are involved in bonding, i.e. there are no lone pairs. However, the arrangement of these electron pairs, and thus the bonded atoms, about each carbon is not even
approximately tetrahedral. Rather, the H-C-H and H-C-C bond angles are much closer to 120°, the
angle which would be expected if three electron pairs were separated in the optimal arrangement,
as just discussed for B Cl3.
This observed geometry can be understood by re-examining the Lewis structure. Recall that,
although there are four electron pairs about each carbon atom, two of these pairs form a double
bond between the carbon atoms. It is tempting to assume that these four electron pairs are forced
apart to form a tetrahedron as in previous molecules. However, if this were this case, the two pairs
involved in the double bond would be separated by an angle of 109.5° which would make it
impossible for both pairs to be localized between the carbon atoms. To preserve the double bond,
we must assume that the two electron pairs in the double bond remain in the same vicinity. Given
this assumption, separating the three independent groups of electron pairs about a carbon atom
produces an expectation that all three pairs should lie in the same plane as the carbon atom,
separated by 120° angles. This agrees very closely with the observed bond angles. We conclude
that the our model can be extended to understanding the geometries of molecules with double (or
triple) bonds by treating the multiple bond as two electron pairs confined to a single domain. It is for this reason that we refer to the model as Electron Domain theory.
Applied in this form, Electron Domain theory can help us understand the linear geometry of CO 2.
Again, there are four electron pairs in the valence shell of the carbon atom, but these are grouped
into only two domains of two electron pairs each, corresponding to the two C=O double bonds.
Minimizing the repulsion between these two domains forces the oxygen atoms to directly opposite
sides of the carbon, producing a linear molecule. Similar reasoning using Electron Domain theory
as applied to triple bonds correctly predicts that acetylene, HCCH, is a linear molecule. If the electron pairs in the triple