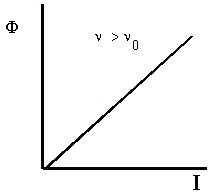Argon is still less than that of Neon, even though the nuclear charge in an Argon atom is much
greater than the nuclear charge in a Neon atom. What have we omitted from our analysis?

The answer is that we must consider also the distance of the electrons from the nucleus. Since it
requires much less energy to ionize a Sodium atom than to ionize a Neon atom even though
Sodium's nuclear charge is greater, it must be that the electron which we remove from a Sodium
atom is much farther from the nucleus than the electron in the Neon atom. We can make the same
comparison of the electrons removed during ionization of Neon and Argon atoms: the Argon
electron must be farther from the nucleus than the Neon electron.
On the other hand, since the ionization energy fairly smoothly increases as we move from Lithium
to Neon in the second period of elements, this reveals that the electrons are increasingly attracted
to the nucleus for greater nuclear charge and suggests that the electrons' distance from the nucleus
might not be varying too greatly over the course of a single period of the table.
If we follow this reasoning, we can even estimate how far an electron might typically be from the
nucleus by using our crude approximation that the ionization energy is equal to the negative of the
Coulomb potential and solving for r for each atom. This gives an estimate of distance of the
electron from the nucleus:
(3.3)
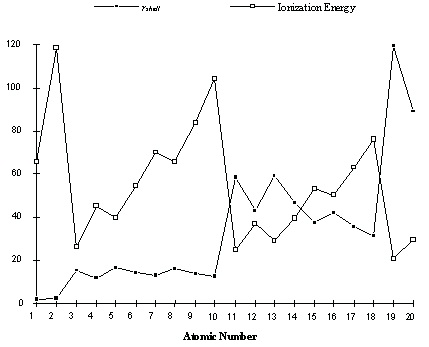
Values of r shell calculated in this way are shown for the first 20 elements here. Also shown for comparison is the ionization energy for these elements. Notice that the approximate distance of
the electrons from the nucleus increases in steps exactly coinciding with the increases and dips in
the ionization energy.
Figure 3.4.
Although these distances we have calculated do not have a precise physical meaning, this figure
suggests a significant conclusion. The electrons in the elements are arranged into "shells" of increasingly greater distance from the nucleus. Hydrogen and Helium, with one and two electrons,
have ionization energies consistent with electrons at similar and close distance from the nucleus.
Then the second row elements lithium through neon have virtually identical sizes, though larger
than that for the first two elements. The third row elements, sodium argon, have an approximate
electron-nuclear distance which fluctuates a bit but is consistently larger than the second row
elements.
Because the sizes of the atoms appear to grow in steps which correspond exactly to the periods of
the Periodic Table, it seems that the electrons in the atoms are grouped into sets which are
differing distances away from the nucleus. The first two electrons, as in Helium, are close to the
nucleus, whereas additional electrons, as in Lithium to Neon, are farther from the nucleus than the
first two. The suggests that, for atoms Lithium to Neon, the first two electrons are in an inner
"shell", and the remaining electrons are in an outer "shell."
We can refine this shell model for the electrons in an atom with further analysis of ionization
energies. We can remove any number of electrons in sequence, forming ions with greater charge.
We have been examining the first ionization energy, IE1 , but each successively removed electron
has successively greater ionization energy:
First ionization energy IE1 :
(3.4)
A (g) → A+ (g) + e– (g)
Second ionization energy IE2 :
(3.5)
A+ (g) → A 2+ (g) + e– (g)
Third ionization energy IE3 :
(3.6)
A 2+ → A 3+ (g) + e– (g)
The sequential ionization energies for the elements in the second row of the periodic table are
shown here.
Table 3.1. Successive Ionization Energies (kJ/mol)
Na
Mg
Al
Si
P
S
Cl
Ar
IE1 496
738
578
787
1012
1000
1251
1520
IE2 4562 1451 1817 1577 1903 2251 2297 2665
IE3 6912 7733 2745 3231 2912 3361 3822 3931
IE4 9543 10540 11575 4356 4956 4564 5158 5770
IE5 13353 13630 14830 16091 6273 7013 6542 7238
IE6 16610 17995 18376 19784 22233 8495 9458 8781
IE7 20114 21703 23293 23783 25397 27106 11020 11995
Note that the second ionization energy is always greater than the first, and the third is always
greater than the second, etc. This makes sense, since an electron should be more strongly attracted
to a positively charged atom than to a neutral atom.
However, the data in the table show a surprising feature. In most cases, the ionization energy increases a fairly large amount for successive ionizations. But for each atom, there is one much
larger increase in ionization in the sequence. In Na for example, IE2 is nearly 10 times greater
than IE1 . Similarly, IE3 is five times greater than IE2 for Mg, although IE2 is less than twice IE1 .
The data for Na through S all show a single large step in addition to the smaller increases in IE.
Looking closely and counting electrons, we see that this unusually large increase always occurs
for the ionization where we have already removed all of the outer shell electrons and are now
removing an electron from the inner shell. This occurs uniformly across the second row elements,
indicating that our shell model is in fact a very accurate predictor of the higher ionization
energies. We can now tell how many electrons there are in the outer shell of each atom: it is equal
to the number of electrons since the last inert gas.
We can conclude that an inner shell is "filled" once we have the number of electrons equal to the number in an inert gas atom. The subsequent electrons are added to a new outer shell. This is
commonly referred to as the valence shell of the atom.
However, we do not know why only a limited number of electrons can reside in each shell. There
is no obvious reason at this point why all the electrons in an atom do not reside in the shell closest
to the nucleus. Similarly, there is no reason given for why the number of electrons in an inert gas
atom exactly fills the outer shell, without room for even a single additional electron. These
questions must be addressed further.
Review and Discussion Questions
Exercise 1.
Explain how the scattering of α particles from gold foil reveals that an atom contains a massive,
positively charged nucleus whose size is much smaller than that of the atom.
Exercise 2.
Explain the significance of the relationship between the frequency of x-ray emission from each
atom and the atomic ranking of that atom in the periodic table.
Exercise 3.
Provide experimental evidence which reveals that the electrons in an atom are grouped into a
valence shell and inner shell electrons.
Exercise 4.
State and explain the evidence which reveals that the outer shell of each inert gas atom is full.
Exercise 5.
Why does the ionization energy for each successive ionization increase for every atom? Why is
the increase from IE4 to IE5 in Si much larger than any of the other increases for Si?
Solutions
Chapter 4. Quantum Energy Levels In Atoms
Foundation
The atomic molecular theory provides us a particulate understanding of matter. Each element is
characterized as consisting of identical, indestructible atoms with atomic weights which have been
determined. Compounds consist of identical molecules, each made up from a specific number of
atoms of each of the component elements. We also know that atoms have a nuclear structure,
meaning that all of the positive charge and virtually all of the mass of the atom are concentrated
in a nucleus which is a very small fraction of the volume of the atom. Finally, we know that the
electrons in the atom are arranged in "shells" about the nucleus, with each shell farther from the nucleus that the previous. The electrons in outer shells are more weakly attached to the atom than
the electrons in the inner shells, and only a limited number of electrons can fit in each shell.
Goals
The shell model of the atom is a good start in understanding the differences in the chemical
properties of the atoms of different elements. For example, we can understand the periodicity of
chemical and physical properties from our model, since elements in the same group have the same
number of electrons in the valence shell.
However, there are many details missing from our description. Other than a very crude calculation
of "distance" of the shells from the nucleus, we have no description of what the differences are between the electrons in different shells. What precisely is a "shell?"
Most importantly, the arrangement of elements into groups and the periodicity of chemical
properties both depend on the concept that a shell is "filled" by a certain number of electrons.
Looking at the number of elements in each period, the number of electrons which fills a shell
depends on which shell is being filled. In some cases, a shell is filled by eight electrons, in others,
it appears to be 18 electrons. What determines how many electrons can "fit" in a shell? Why is there a limit at all?
Finally, a closer look at the ionization energies here reveals that our shell model must be incomplete. Our model implies that the elements of the second period from Lithium to Neon have
their valence electrons in the second shell. With increasing nuclear charge, the ionization energy
of these atoms should increase from Lithium to Neon. As a general trend, this is true, but there are
variations. Note that the ionization energy of Oxygen atoms is less than that of Nitrogen atoms.
We need to pursue additional detail in our model of the structure of the atom.
Observation 1: The Spectrum of Hydrogen


To begin, we need to know a little about light. All forms of electromagnetic radiation travel as an
oscillating wave, with an electric field component perpendicular to a magnetic field component.
As a wave, the radiation can be characterized by its "wavelength", symbolized as λ, which is the distance between adjacent peaks in the wave. Different wavelengths correspond to different forms
of electromagnetic radiation. For example, microwave radiation has wavelength in the range of
10-2 to 10-3 meters, whereas x-ray radiation has wavelength in the range 10-9 to 10-10 meters.
Radiation which is visible to the human eye has wavelength in the very narrow range from
3.8×10-7 to 7.8×10-7 meters.
Radiation can also be characterized by the frequency of the electromagnetic wave, which is the
number of peaks in the wave which pass a point in space per second. Frequency is symbolized by
ν. The speed which light travels in a vacuum in the same for all forms of electromagnetic
radiation,
. As such, we can relate the frequency of light to the wavelength of light by
the equation
(4.1)
The longer the wavelength λ, the lower the frequency ν. This makes sense when we remember that light travels at a fixed speed. When the wavelength is longer, fewer peaks will pass a point in
space in a second. From this equation, there is a specific relationship between frequency and
wavelength, and either or both can be used to characterize the properties of radiation.
With this background in hand, we can use our understanding of light to pursue more data about the
energies of electrons in atoms. Ionization energies tell us how much energy is required to remove
an electron from an atom, but do not tell what happens if an electron changes its energy in an
atom. To analyze this, we need a means to measure the energies gained or lost by an atom. One
way to do so is to analyze the "spectrum" of an atom, which is the set of frequencies of light emitted by the atom. Since hydrogen is the simplest atom, we analyze the hydrogen spectrum first.
We find that, if we pass a current of electricity through a sample of hydrogen gas, light is emitted.
Careful analysis shows that, although some of this light is emitted by H 2 molecules, some of the light is also emitted by H atoms. Since light is a form of energy, then these H atoms must release
energy supplied to them by the electrons in the current.
Most importantly, if we pass the light emitted by the hydrogen gas sample through a prism, we
can separate the colors as in a rainbow, each with a characteristic frequency. The resultant image
of separated colors is called the spectrum of hydrogen. We find in this experiment that there are only four frequencies (four colors) of light in the emission that are visible. The most intense of the
lines in the spectrum is bright red, but there are blue and violet lines. It turns out that there are
also many other frequencies of light emitted which are invisible to the human eye.
Careful observation and analysis reveals that every frequency in the hydrogen atom spectrum can
be predicted by a very simple formula, called the Rydberg equation:

(4.2)
where R is the Rydberg constant ( 3.29×1015 s-1 ). n and m are integers (1,2,3,...). Each choice of n and m predicts a single observed frequency in the hydrogen atom spectrum.
The atoms of all elements emit radiation when energized in an electric current, and as do all
molecules of all compounds. However, we find that the specific frequencies of light emitted are
characteristic of each atom or molecule. In other words, the spectrum of each element is unique to
each element or compound. As a result, the spectrum of each substance can be used to identify
that substance. (Note that the Rydberg equation tells us only the spectrum of hydrogen.)
Our interest is in the fact that the radiation emitted by an atom tells us about the amounts of
energy which can be released by an atom. For a hydrogen atom, for example, these changes in
energy must correspond to the amounts of energy which the electrons inside the atom can gain or
lose.
At this point, we need to relate the frequency of radiation emitted by an atom to the amount of
energy lost by the electron in the atom. We thus examine some observations about the energy of
radiation.
Observation 2: The Photoelectric Effect
When a light source is directed at a metal surface, it is found under many circumstances that
electrons are ejected from the surface. This phenomenon is called the "photoelectric effect." These electrons can be collected to produce a usable electric current. (This effect has a variety of
common practical applications, for example, in "electric eye" devices.) It is reasonable to expect that a certain amount of energy is required to liberate an electron from a metal surface, since the
electron is attracted to the positively charged nuclei in the metal. Thus, in order for the electron to
escape, the light must supply sufficient energy to the electron to overcome this attraction.
The following experimental observations are found when studying the photoelectric effect. First,
in order for the effect to be observed, the light must be of at least a minimum frequency which we
call the threshold frequency, ν 0 . This frequency is a characteristic for a given metal. That is, it is the same value for each sample of that metal, but it varies from one metal to the next. For low
frequency light, photoelectrons are not observed in any number, no matter how intense the light
source is. For light with frequency above ν 0 , the number of photoelectrons emitted by the metal (measured by the photoelectric current, Φ) increases directly with the intensity of the light. These results are shown in Figure 4.1.
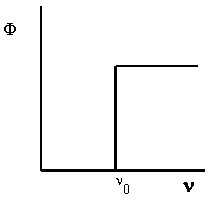
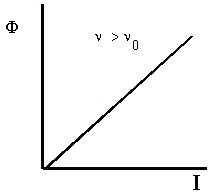
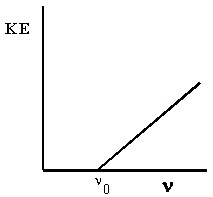
(a) For photoelectrons to be emitted, the light frequency must (b) If the frequency is high enough, the number of photoelectrons be greater than a threshold value.
increases directly with the light intensity.
Figure 4.1. The Photoelectric Effect
Φ is the photoelectric current, ν is the frequency of incident light, and I is the intensity of incident light.
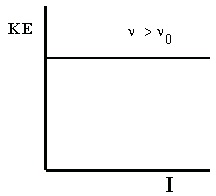
Second, we can measure the energies of the electrons emitted by the metal. For a given metal, all
photoelectrons have the same kinetic energy for a fixed frequency of light above ν 0 . This fixed kinetic energy is independent of the intensity of the light source. As the frequency of the light is
increased, the kinetic energy of the emitted electrons increases proportionally. These results are
shown in Figure 4.2.
(a) If the frequency is high enough, the energy of the electrons increases (b) However, the energy of the photolectrons does not directly with the frequency.
depend on the light intensity.
Figure 4.2. More Photoelectric Effect
KE is the photoelectron kinetic energy, ν is the frequency of incident light, and I is the intensity of incident light.
Are these results surprising? To the physicists at the end of the nineteenth century, the answer was
yes, very surprising indeed. They expected that the energy of the light source should be
determined by its intensity. Hence, the energy required to eject a photoelectron should be supplied
by light of high intensity, no matter how low the frequency of the radiation. Thus, there should be
no threshold frequency, below which no electrons are emitted. Moreover, the kinetic energy of the
electrons should increase with intensity, not with light frequency. These predictions are not
observed, so the results are counter to physical intuition.
We can account for these results in a straightforward but perhaps non-obvious manner. (Einstein
provided the explanation in 1905.) Since the kinetic energy of the emitted photoelectrons
increases proportionally with increases in the frequency of the light above the threshold
frequency, we can conclude from conservation of total energy that the energy supplied by the light
to the ejected electron must be proportional to its frequency: ( E ∝ ν) . This does not immediately account for the existence of the threshold frequency, though, since it would still seem to be the
case that even low frequency light would possess high energy if the intensity were sufficient. By
this reasoning, high intensity, low frequency light should therefore produce as many
photoelectrons as are produced by low intensity, high frequency light. But this is not observed.
This is a very challenging puzzle, and an analogy helps to reveal the subtle answer. Imagine trying
to knock pieces out of a wall by throwing objects at it. We discover that, no matter how many ping
pong balls we throw, we cannot knock out a piece of the wall. On the other hand, only a single
bowling ball is required to accomplish the task. The results of this "experiment" are similar to the observations of the photoelectric effect: very little high frequency light can accomplish what an
enormous amount of low frequency light cannot. The key to understanding our imaginary
experiment is knowing that, although there are many more ping-pong balls than bowling balls, it
is only the impact of each individual particle with the wall which determines what happens.
Reasoning from this analogy, we must conclude that the energy of the light is supplied in
"bundles" or "packets" of constant energy, which we will call photons. We have already concluded that the light supplies energy to the electron which is proportional to the light
frequency. Now we can say that the energy of each photon is proportional to the frequency of the
light. The intensity of the light is proportional to the number of these packets. This now accounts
for the threshold frequency in a straightforward way. For a photon to dislodge a photoelectron, it
must have sufficient energy, by itself, to supply to the electron to overcome its attraction to the
metal. Although increasing the intensity of the light does increase the total energy of the light, it
does not increase the energy of an individual photon. Therefore, if the frequency of the light is too
low, the photon energy is too low to eject an electron. Referring back to the analogy, we can say
that a single bowling bowl can accomplish what many ping-pong balls cannot, and a single high
frequency photon can accomplish what many low frequency photons cannot.
The important conclusion for our purposes is that light energy is quantized into packets of
energy. The amount of energy in each photon is given by Einstein’s equation,
(4.3)
E= hν
where h is a constant called Planck’s constant.
Quantized Energy Levels in Hydrogen Atoms
We can combine the observation of the hydrogen atom spectrum with our deduction that light
energy is quantized into packets to reach an important conclusion. Each frequency of light in the

spectrum corresponds to a particular energy of light and, therefore, to a particular energy loss by a hydrogen atom, since this light energy is quantized into packets. Furthermore, since only certain
frequencies are observed, then only certain energy losses are possible. This is only reasonable if
the energy of each hydrogen atom is restricted to certain specific values. If the hydrogen atom
could possess any energy, then it could lose any amount of energy and emit a photon of any
energy and frequency. But this is not observed. Therefore, the energy of the electron in a hydrogen
atom must be restricted to certain energy levels.
The Hydrogen atom spectrum also tells us what these energy levels are. Recall that the
frequencies of radiation emitted by Hydrogen atoms are given by the Rydberg equation. Each choice of the positive integers n and m predicts a single observed frequency in the hydrogen atom spectrum.
Each emitted frequency must correspond to an energy hν by Einstein’s equation. This photon energy must be the difference between two energy levels for a hydrogen electron, since that is the amount of energy released by the electron moving from one level to the other. If the energies of
the two levels are Em and En, then we can write that
(4.4)
hν= Em− En
By comparing this to the Rydberg equation, each energy level must be given by the formula
(4.5)
We can draw two conclusions. First, the electron in a hydrogen atom can exist only with certain
energies, corresponding to motion in what we now call a state or an orbital. Second, the energy of a state can be characterized by an integer quantum number, n = 1, 2, 3, ... which determines its
energy.
These conclusions are reinforced by similar observations of spectra produced by passing a current
through other elements. Only specific frequencies are observed for each atom, although only the
hydrogen frequencies obey the Rydberg formula.
We conclude that the energies of electrons in atoms are "quantized," that is, restricted to certain values. We now need to relate this quantization of energy to the existence of shells, as developed
in a