19. Most naturally abundant elements have,
a) high electronegativity,
b) higher melting points,
c) higher nuclear charge,
d) higher nuclear binding energy.
20. As the atomic mass number increases, the nuclear binding energy,
a) increase,
b) increases then decreases,
c) remains constant,
d) increases then remains constant.
Answer key
1.c 2. c 3. a 4. d 5. c 6. b, 7. d, 8. c, 9. a, 10. d, 11.b, 12. a, 13. c, 14. c, 15. c, 16.
d, 17. a, 18. b, 19. d, 20. b

African Virtual University
Learning activity # 4
Title of Learning Activity : OCCURREnCE, ABUndAnCE
And EXTRACTIOn
At the end of this Unit, the learner should be able to
1. Appreciate the natural abundances of the elements.
2. State, predict, and explain the methods and ways of extraction of sblock ele-
ments
3. State, predict, and explain the general methods and of exctractions of the
metallic p-block elements according the electrochemical series.
4. State, predict, and explain the general methods of extraction of non-metallic
p-block elements.
5. State, predict and explain the general methods of extraction and purification
of the p-block geaseous elements.
6. Carry out a research project based on the extraction of some s- and p-block
elements and write a report.
Summary of the learning activity
So far we have considered the positions and trends in properties for the s-, and p-
block elements of the periodic table. The current unit braches to occurences, abun-
dance and extractions methods compounds of the s-, and p-block elements. The unit
will consider the elements as a group in the periodic table with a few representative
members of the group considered. A general extraction trend, using the position of
the element in the electrochemical series, will be appreciated. The extraction of the
gaseous elements especially in the atmosphere will also be discussed.
List of Required Readings
1. Alan G. Sharpe; Inorganic Chemistry, 3rd Edition. Longman Singapore Pu-
blisher (1992).
2. William L. Jolly, Modern inorganic Chemistry 2nd Ed. McGraw-Hill. Inc.
New York, USA (1991).
3. Catherine E. Housecroft and Alan G. Sharpe; Inorganic Chemistry. Prentice-
Hall International, USA. (2000).
4. F. Albert Cotton and Geoffrey Wilkinson, F. R. S. Advanced Inorganic Che-
mistry, A Comprehensive text, 6th edition. Wiley Eastern Limited, New Delhi.
India. (1984).
5. J. D. Lee, Concise Inorganic Chemistry, 4th edition. Chapman & Hall, New
York. USA. (1993).

African Virtual University
6. Thomas R. Gilbert, Rein V. Kirss, and Geoffrey Davies; Chemistry, The
science in context. W.W. Norton and company NY, USA.(2004).
7. Carole H. McQuarrie, Donald A. McQuarrie and Peter A. Rock. General Che-
mistry, 3rd edition. W. H. Freeman and Company, New York, USA, (1990).
List of relevant useful links
http://www.infoplease.com/ce6/sci/A0859587.html Useful for the introduction
to metal extractions
http://www.citycollegiate.com/sblock1.htm
http://www.wissensdrang.com/auf1na1.htm
www.citycollegiate.com . For free computer courses and notes
http://en.wikipedia.org/wiki/Frasch_process For sulphur process
http://www.chemsoc.org/viselements/pages/fluorine.html details on flourine
List of relevant MULTIMEDIA resources
- Computer with internet connecting facility to access relevant links and free
source resourses.
- Multi-media resourses such as CD players, VCD etc.
- CD-ROM for this module for compulsory reading and demonstrations.
Learning activities
Occurrence, Abundance and Extraction.
A solid element or compound which occurs naturally in the Earth’s crust is called a
mineral. A mineral which contains a high enough percentage of a metal for economic
extraction is called a metal ore. The most common metal ores are oxides and sulphides.
Sulphides are the oldest ores, formed in the Earth’s history when there was a lot of
sulphur from volcanic activity. Oxides formed later when photosynthesis in plants
released large amounts of oxygen into the atmosphere. Metal ore deposits are a finite
resource (there are only a certain amount of them) and non-renewable. Many metals
are obtained today from recycling (melting and refining) scrap metals. About half
of the aluminium, copper, lead, steel and tin which are used in the UK today come
from recycled scrap metal.
A metal above carbon in the reactivity series may be extracted from its ore by elec-
trolysis. A metal below carbon in the reactivity series (zinc to silver) may be extrac-
ted from its ore by heating with carbon. The metal is displaced from its non-metal
anion by the more reactive carbon. Carbon is used because it is readily available and
cheap (coke or charcoal are both carbon). The metal in the ore is said to be reduced
by reaction with carbon. Hydrogen may be used to reduce metals which are lower
than itself in the reactivity series, but since it is more expensive than carbon it is

African Virtual University
only used on a large scale for the extraction of tungsten, to avoid the formation of
tungsten carbide.
Below is a general summary of the extraction methods and where they are applica-
ble.
Method
Type of metal
Mining the pure metal
Noble metals such as Gold, Silver, and
platinum
Roasting the sulphide, and reduction
Mostly metals of the p-block elements
of the oxides
Reduction of the oxides
Less reactive especially in the p-block
elements
Electrolysis of the molten solids
Reactive elements in Groups 1, 2, & 3,
eg Na, Mg, and Al.
Alkali Metals
Despite being close family members, the elements of group 1 do not occur together
largely because of their different ionic sizes. These elements are too reactive to be
found free in nature. Sodium occurs mainly as NaCI (salt) in sea-water and dried-up
sea beds. Potassium is more widely distributed in minerals such as sylvite, KCI, but
is also extracted from sea-water. The alkali metals are so reactive they cannot be
displaced by another element, so are isolated by electrolysis of their molten salts
Lithium is the thirty-fifth most abundant element in the earth crust (approximately
18 ppm). Sodium and potassium are in numbers seven (approximately 22 700 ppm)
and eight (approximately 18 400 ppm) respectively. Rb and Cs are mainly obtained
as by-products from Lithium processing. Francium is radioactive and does not occur
appreciably in nature.
Extraction
The compounds of group 1 are very stable to heat and very high in electronegative
series and they react with water, so both thermal decomposition and displacement by
another element in solution are impractical. The metals are also very strong chemical
reducing agents, meaning that they can not be replaced by reducing their oxides.
Also, electrolysis of aqueous solutions in order to obtain the element may only be
successful if mercury cathode is used.
Thus the metals are all isolated by electrolysis of their fused salts, mostly halides,
and often with impurities added to lower the melting point.

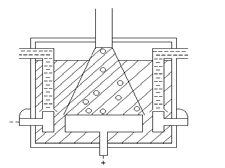
African Virtual University
The Electrolytic Production of Lithium
Electrolyte LiCl–KCl eutectic feed LiCl temperature 400–460oC. Anode carbon
cathode Mild Steel
Overall Cell Reaction LiCl (l) ➔Li (l) + 1/2 Cl (g) for which E
= 3.6 V
2
427oC
Anode Cl– ➔1/2 Cl (g) + e–
2
Cathode Li+ + e– ➔Li (l)
Current Density 2 A/cm2
Energy Consumption 35 kWh/kg
Extraction of sodium
On industrial scale sodium metal is extracted by “Down’s Process”. Sodium is extrac-
ted by the electrolytic reduction of purified, molten rock salt. Down’s cell consists of
a rectangular container of steel. Inside of the tank is lined with firebricks. Anode is
a graphite rod which projects centrally up through the base of the cell. Cathode is a
ring of iron, which surrounds the anode. The anode and cathode are separated from
each other by a cylindrical steel gauze diaphragm so that Na and Cl are kept apart.
2
A bell like hood is submerged over the anode.
The main draw back is that the Melting point of NaCl is 801oC. At this temperature,
molten NaCl and Na form a metallic fog in the container, which is impossible to
separate.
In order to over come this difficulty instead of only NaCl, a mixture of NaCl (42%)
and CaCl (58%) is electrolyzed in down’s cell. The melting point of this mixture is
2
600C. At 600oC no metallic fog is formed.
When an electric current is passed through the molten mixture of NaCl and CaCl ,
2
NaCl decomposes in to Na+ and Cl- ion. Na+ ions migrate towards cathode while Cl-
ions towards the anode. The molten sodium collects in the cathode compartment where
it rises to the top and is tapped off by a pipe. Chlorine is collected at the anode.
Label this diagram of a Downs electrolytic cell with: Carbon anode; Chlorine gas;
Insulator; Molten electrolyte; Molten sodium; and, Steel cathode.

African Virtual University
The chemistry.
Fused NaCl contains sodium and chloride ions.
2NaCl ➔2Na+ + 2Cl-
Electrochemical changes
At cathode
Na+-ions migrate to cathode where they are reduced to Na.
2Na+ + 2e- ➔2Na (Reduction)
At anode
Cl--ions migrate to anode and oxidised to form chlorine gas.
2Cl- ➔Cl + 2e- (Oxidation)
2
Overall Reaction
2Na+ + 2e- ➔2Na
2Cl- ➔Cl + 2e-
2
__________________
2Na+ + 2Cl- ➔2Na + Cl 2
After manufacture, sodium is stored under oil because it is rapidly oxidized by atmos-
pheric oxygen. Furthermore, sodium reacts explosively with many other non-metallic
elements and compounds.
A similar cell can be used for the extraction of Potasium from KCl, though the cell
must be operated at higher temperatures. Modern method uses cheaper Sodium vapour
to reduce the K at 850oC in large floatation tanks.
Na + KCl ➔ NaCl + K

African Virtual University
Question Explain why during electrolysis calcium is also obtained but it does not
mix with Sodium?.
Answer: During electrolysis calcium is also obtained at cathode but sodium and
calcium are separated from each other due to difference in density. Density of Na is
0.67gm/cc and the density of Ca is much higher than that of Na i.e. 2.54gm/cc. That’s
why they do not mix with each other.
Alkali earth metals
Occurrence and Extraction
These elements are all found in the Earth’s crust, but not in the elemental form as
they are so reactive. Instead, they are widely distributed in rock structures. The main
minerals in which magnesium is found are carnellite, magnesite and dolomite. Cal-
cium is found in chalk, limestone, gypsum and anhydrite. Magnesium is the eighth
most abundant element in the Earth’s crust, and calcium is the fifth.
Industrial Information
Magnesium is the only Group 2 element used on a large scale. It is used in flares,
tracer bullets and incendiary bombs as it burns with a brilliant white light. It is also
alloyed with aluminium to produce a low-density, strong material used in aircraft.
Magnesium oxide has such a high melting point it is used to line furnaces.
Two methods of producing magnesium commercially are used. The principal method
is the dow Sea water process; electrolysis of fused magnesium chloride, which is
used in the extraction of magnesium from seawater (the principal source) and from
dolomite. In recovery from seawater, the magnesium is precipitated as magnesium
hydroxide by treatment with lime (calcium oxide) obtained from oyster shells. The
hydroxide is collected and treated with hydrochloric acid to form the chloride. The
chloride is fused and electrolyzed, forming magnesium metal and chlorine gas. The
molten metal is cast into ingots for further processing; the chlorine gas is made into
hydrochloric acid and is reused to form magnesium chloride. About 1 lb of magnesium
is recovered from each 100 gal of seawater; the oceans are a virtually inexhaustible
source of this metal (0.13% Mg). A second method of magnesium production, called
the ferrosilicon process, involves the reduction of magnesium oxide (prepared by
calcining dolomite) with an iron-silicon alloy.
All the factors that were considered in choosing the extraction method for Group 1
metals are also relevant here. All the metals can be obtained by electrolysis of their
fused chloride, with sodium chloride added to lower the melting point.
(Write the chemical equation for the reaction described above).

African Virtual University 0
Group 13
Occurrence and Extraction
These elements are not found free in nature, but all are present in various minerals and
ores. The most important aluminium-containing minerals are bauxite and cryolite.
Aluminium is the most widely used element in this Group. It is obtained by the elec-
trolysis of aluminium oxide, which is purified from bauxite. The melting point of
the aluminium oxide is too high for electrolysis of the melt, so instead it is dissolved
in molten cryolite.
Industrial Information
Boron.
Discovered : by J.L. Gay-Lussac and L.J. Thenard in Paris, France, and Sir Humphry
Davy in London, UK in 1808. The name is derived from the Arabic ‘buraq’, borax,
its principal ore. Pure boron is a little-used dark powder, but boron compounds are
important in many industries, such as glass and detergent manufacture and agriculture.
Pyrex glass is tough and heat resistant because of the boric acid used to make it. Boron
is an essential mineral for plants but not animals - in fact it can be toxic in excess. We
take in about 2 milligrams each day from our food (about 60g in a lifetime).
Boron of low quality is obtained by reducing the B O with Mg and Na at high
2
3
temperatures. Pure Boron is not easy to obtain as it has very high temperatures of
2180oC.
Aluminum
Aluminium is obtained from its Bauxite ore; which may be AlO.OH.(Al O .H O)
2
3
2
or Al(HO) .(Al O .3H O). The ore is first purified by removing the waste (usually
3
2
3
2
Iron and silicon compounds) by dissolving the ore in NaOH. The amphoteric Al
dissolves but not the impurities. Then the Al(OH) is precipitated from the alkaline
3
aluminate either by bubbling through the solution CO gas or by seeding the solution
2
with Al O . The Al(OH) is then calcined (heated strongly) to produce pure Al O .
2
3
3
2
3
Al O is then melted with cryolite Na [AlF ] and electrolyzed in graphite lined steel
2
3
3
6
tank, which also serves as the cathode. The anodes are all made of graphite.The cell
runs continuously, and at intervals molten aluminium (m.p 600oC) is tapped from the
bottom of the cell and more bauxite is added.

African Virtual University
Quiz 1. What are the uses of the cryolite?
Solution 1 it improves the electrical conductivity of the cell as Al O is a poor 2
3
conductor and 2) it serves as added impurity and lowers the melting point of the
mixture to about 950oC.
Quiz 2. Find the products formed at the anode terminal of the cell.
Solution: O , CO , and F .
2
2
2
Quiz. 3.what is the main draw back of this process?.
Solution 3. It is energy intensive and hence is only economical where cheap hydroe-
lectricity power is available.
Group 14
The group 14 elements are well known except for germanium, carbon is the seven-
teenth, and silicon is the second most abundant element by weight in the earth crust.
Ge, too, occurs as traces in mineral ores. Though the abundance of Tin and Lead are
comparatively low, they occur in concentrated deposits and hence easy to extract.
Carbon occurs in deposits as Coal, Crude oil, and carbonate salts. It similarly occurs
as graphite, diamond, soot, CO , and even as CO gas.
2
Coke is produced in large amounts by high temperature carbonization of coal. Here
the coal is heated in large ovens in the absence of air. The Coke is extensively used
in the metallurgical extraction of iron and many other metals.
Quiz. How would diamond and graphite be extracted?.
Solution. For diamond and graphite, they are mined then just purified/cleaned as they
occur ‘native’-uncombined in most cases.
Silicon
The elemental Si is obtained by reducing the SiO with excess high purity coke.
3
Though high purity Si is obtained by converting the Si to SiCl , purifying this by
4
distillation, and then reducing the chloride with Mg or Zn.
SiO + xC ➔ Si + 2CO
2
Si + yCl ➔ SiCl
2
4

African Virtual University
SiCl + zMg ➔ Si + 2MgCl
4
2
n.B. Kindly balance the equations above by getting the numerical values (coefficients)
x, y, and z. (x = y = x = 2).
Semiconductor quality Si can be made by sodium reduction of Na [SiF ].
2
6
Under exposure to oxygen, a silicon surface oxidizes to form silicon dioxide (SiO ).
2
Native silicon dioxide is a high-quality electrical insulator and can be used as a barrier
material during impurity implants or diffusion, for electrical isolation of semiconduc-
tor devices, as a component in transistors, or as an interlayer dielectric in multilevel
metallization structures such as multichip modules. The ability to form a native oxide
was one of the primary processing considerations which led to silicon becoming the
dominant semiconductor material used in integrated circuits today.
n.B. Kindly, get the extraction process for Lead (from the ore galena) and Tin (Cas-
siterite ore).
Group 15
Nitrogen comprises 78% of the earth’s atmosphere, though not very abundant in the
earth crust. This could be partly because nitrates are very soluble in water hence not
‘stable’ in the crust, though a few are found in desert deposits (NaNO ) like in Chile.
3
Phosphorus is the eleventh most abundant element in the earth crust. It also constitutes
60% our human beings bones and teeth. Mostly it is found as flouroapatite [Ca (PO ) .
3
4 2
CaF ] and hydroxyapatite [Ca (PO ) .Ca(OH) ]. The elements of As, Sb and Bi are
2
3
4 2
2
not very abundant. Their most important source is the sulphides occurring as traces
in mineral ores. Care should be taken as both As and Sb are poisonous.
nitrogen
Condensing air to liquid state, and then fractionally distilling the liquid air commer-
cially obtain the nitrogen gas. Since, nitrogen has a lower boiling point than O , it
2
distills off first.
Quiz 1. What are the major components of liquid air?.
Answer: N , O , Ar, H O, CO , Ne, H , He, etc.
2
2
2
2
2
Quiz. 2. What are the boiling points for liquid N , O , CO , and H O?.
2
2
2
2

African Virtual University
Answer: N ,(-196 oC) O (-183 oC), H O (0 oC), CO (-78 oC sublimes).
2
2
2
2
Phosphorus
Found most often in phosphate rock. Primary mining areas are Russia, USA, Morocco,
Tunisia, Tongo and Nauru. Commercially obtained by reducing the calcium phos-
phate with C in an electric furnace at 1400 – 1500oC. Sand (silica SiO2) is added to
remove the calcium as liquiod slag (calcium silicate) and to drive off the phosphorus
as P O . Carbon reduces the P O to P. At higher temperatures, gaseous P distills
4
10
4
10
off, mainly as P but with some P . This is then condensed to white phosphorus by
4
2
passing it through water.
2Ca (PO ) + 6SiO ➔ 6CaSiO + P O
3
4 2
2
3
4
10
P O + 10C ➔ P + 10CO
4
10
4

African Virtual University
Group 16
Oxygen is the most abundant of all the elements. In free from, it forms 21 % by
composition but 23 % by weight of the earth’s atmosphere. However, oxygen makes
up 47 % of the earth crust where its major component is the silicate mineral, oxides,
sulphates and carbonates. It makes 89 % by weight of the world ocean waters .
Sulfur is the sixteenth most abundant element and it constitutes 0.03%















