Figure 7.30 Past and projected world energy use (source: Based on data from U.S. Energy Information Administration, 2011)
Figure 7.31 Solar cell arrays at a power plant in Steindorf, Germany (credit: Michael Betke, Flickr)
Table 7.6 displays the 2006 commercial energy mix by country for some of the prime energy users in the world. While non-renewable sources
dominate, some countries get a sizeable percentage of their electricity from renewable resources. For example, about 67% of New Zealand’s
electricity demand is met by hydroelectric. Only 10% of the U.S. electricity is generated by renewable resources, primarily hydroelectric. It is difficult
to determine total contributions of renewable energy in some countries with a large rural population, so these percentages in this table are left blank.
Table 7.6 Energy Consumption—Selected Countries (2006)
Consumption,
Electricity Use
Energy Use
Natural
Other
Country
Oil
Coal
Nuclear
Hydro
per capita (kWh/
per capita (GJ/
in EJ (1018 J)
Gas
Renewables
yr)
yr)
Australia
5.4
34%
17%
44%
0%
3%
1%
10000
260
Brazil
9.6
48%
7%
5%
1%
35%
2%
2000
50
China
63
22%
3%
69%
1%
6%
1500
35
Egypt
2.4
50%
41%
1%
0%
6%
990
32
Germany
16
37%
24%
24%
11%
1%
3%
6400
173
India
15
34%
7%
52%
1%
5%
470
13
Indonesia
4.9
51%
26%
16%
0%
2%
3%
420
22
Japan
24
48%
14%
21%
12%
4%
1%
7100
176
New
0.44
32%
26%
6%
0%
11%
19%
8500
102
Zealand
Russia
31
19%
53%
16%
5%
6%
5700
202
U.S.
105
40%
23%
22%
8%
3%
1%
12500
340
World
432
39%
23%
24%
6%
6%
2%
2600
71
Energy and Economic Well-being
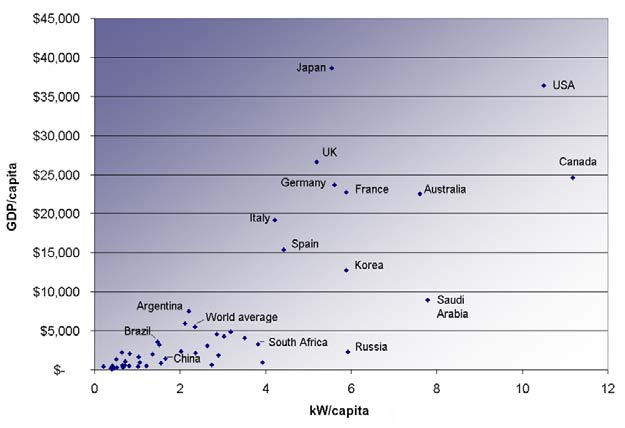
The last two columns in this table examine the energy and electricity use per capita. Economic well-being is dependent upon energy use, and in most
countries higher standards of living, as measured by GDP (gross domestic product) per capita, are matched by higher levels of energy consumption
per capita. This is borne out in Figure 7.32. Increased efficiency of energy use will change this dependency. A global problem is balancing energy
resource development against the harmful effects upon the environment in its extraction and use.
250 CHAPTER 7 | WORK, ENERGY, AND ENERGY RESOURCES
Figure 7.32 Power consumption per capita versus GDP per capita for various countries. Note the increase in energy usage with increasing GDP. (2007, credit: Frank van
Mierlo, Wikimedia Commons)
Conserving Energy
As we finish this chapter on energy and work, it is relevant to draw some distinctions between two sometimes misunderstood terms in the area of
energy use. As has been mentioned elsewhere, the “law of the conservation of energy” is a very useful principle in analyzing physical processes. It is
a statement that cannot be proven from basic principles, but is a very good bookkeeping device, and no exceptions have ever been found. It states
that the total amount of energy in an isolated system will always remain constant. Related to this principle, but remarkably different from it, is the
important philosophy of energy conservation. This concept has to do with seeking to decrease the amount of energy used by an individual or group
through (1) reduced activities (e.g., turning down thermostats, driving fewer kilometers) and/or (2) increasing conversion efficiencies in the
performance of a particular task—such as developing and using more efficient room heaters, cars that have greater miles-per-gallon ratings, energy-
efficient compact fluorescent lights, etc.
Since energy in an isolated system is not destroyed or created or generated, one might wonder why we need to be concerned about our energy
resources, since energy is a conserved quantity. The problem is that the final result of most energy transformations is waste heat transfer to the
environment and conversion to energy forms no longer useful for doing work. To state it in another way, the potential for energy to produce useful
work has been “degraded” in the energy transformation. (This will be discussed in more detail in Thermodynamics.)
Glossary
basal metabolic rate: the total energy conversion rate of a person at rest
chemical energy: the energy in a substance stored in the bonds between atoms and molecules that can be released in a chemical reaction
conservation of mechanical energy: the rule that the sum of the kinetic energies and potential energies remains constant if only conservative
forces act on and within a system
conservative force: a force that does the same work for any given initial and final configuration, regardless of the path followed
efficiency: a measure of the effectiveness of the input of energy to do work; useful energy or work divided by the total input of energy
electrical energy: the energy carried by a flow of charge
energy: the ability to do work
fossil fuels: oil, natural gas, and coal
friction: the force between surfaces that opposes one sliding on the other; friction changes mechanical energy into thermal energy
gravitational potential energy: the energy an object has due to its position in a gravitational field
horsepower: an older non-SI unit of power, with 1 hp = 746 W
joule: SI unit of work and energy, equal to one newton-meter
kilowatt-hour: (kW ⋅ h) unit used primarily for electrical energy provided by electric utility companies
kinetic energy: the energy an object has by reason of its motion, equal to 12 mv 2 for the translational (i.e., non-rotational) motion of an object of
mass m moving at speed v
law of conservation of energy: the general law that total energy is constant in any process; energy may change in form or be transferred from
one system to another, but the total remains the same
CHAPTER 7 | WORK, ENERGY, AND ENERGY RESOURCES 251
mechanical energy: the sum of kinetic energy and potential energy
metabolic rate: the rate at which the body uses food energy to sustain life and to do different activities
net work: work done by the net force, or vector sum of all the forces, acting on an object
nonconservative force: a force whose work depends on the path followed between the given initial and final configurations
nuclear energy: energy released by changes within atomic nuclei, such as the fusion of two light nuclei or the fission of a heavy nucleus
potential energy of a spring: the stored energy of a spring as a function of its displacement; when Hooke’s law applies, it is given by the
expression 1
2 kx 2 where x is the distance the spring is compressed or extended and k is the spring constant
potential energy: energy due to position, shape, or configuration
power: the rate at which work is done
radiant energy: the energy carried by electromagnetic waves
renewable forms of energy: those sources that cannot be used up, such as water, wind, solar, and biomass
thermal energy: the energy within an object due to the random motion of its atoms and molecules that accounts for the object's temperature
useful work: work done on an external system
watt: (W) SI unit of power, with 1 W = 1 J/s
work-energy theorem: the result, based on Newton’s laws, that the net work done on an object is equal to its change in kinetic energy
work: the transfer of energy by a force that causes an object to be displaced; the product of the component of the force in the direction of the
displacement and the magnitude of the displacement
Section Summary
7.1 Work: The Scientific Definition
• Work is the transfer of energy by a force acting on an object as it is displaced.
• The work W that a force F does on an object is the product of the magnitude F of the force, times the magnitude d of the displacement,
times the cosine of the angle θ between them. In symbols,
W = Fd cos θ.
• The SI unit for work and energy is the joule (J), where 1 J = 1 N ⋅ m = 1 kg ⋅ m2/s2 .
• The work done by a force is zero if the displacement is either zero or perpendicular to the force.
• The work done is positive if the force and displacement have the same direction, and negative if they have opposite direction.
7.2 Kinetic Energy and the Work-Energy Theorem
• The net work W net is the work done by the net force acting on an object.
• Work done on an object transfers energy to the object.
• The translational kinetic energy of an object of mass m moving at speed v is KE = 12 mv 2 .
• The work-energy theorem states that the net work W
2
net on a system changes its kinetic energy, W net = 12 mv 2 − 12 mv 0 .
7.3 Gravitational Potential Energy
• Work done against gravity in lifting an object becomes potential energy of the object-Earth system.
• The change in gravitational potential energy, ΔPEg , is ΔPEg = mgh , with h being the increase in height and g the acceleration due to
gravity.
• The gravitational potential energy of an object near Earth’s surface is due to its position in the mass-Earth system. Only differences in
gravitational potential energy, ΔPEg , have physical significance.
• As an object descends without friction, its gravitational potential energy changes into kinetic energy corresponding to increasing speed, so that
ΔKE= −ΔPEg .
7.4 Conservative Forces and Potential Energy
• A conservative force is one for which work depends only on the starting and ending points of a motion, not on the path taken.
• We can define potential energy (PE) for any conservative force, just as we defined PEg for the gravitational force.
• The potential energy of a spring is PEs = 12 kx 2 , where k is the spring’s force constant and x is the displacement from its undeformed
position.
• Mechanical energy is defined to be KE + PE for a conservative force.
252 CHAPTER 7 | WORK, ENERGY, AND ENERGY RESOURCES
• When only conservative forces act on and within a system, the total mechanical energy is constant. In equation form,
KE + PE = constant ⎫
or
⎬
KEi + PEi = KEf + PEf⎭
where i and f denote initial and final values. This is known as the conservation of mechanical energy.
7.5 Nonconservative Forces
• A nonconservative force is one for which work depends on the path.
• Friction is an example of a nonconservative force that changes mechanical energy into thermal energy.
• Work W nc done by a nonconservative force changes the mechanical energy of a system. In equation form, W nc = ΔKE + ΔPE or,
equivalently, KEi + PEi + W nc = KEf + PEf .
• When both conservative and nonconservative forces act, energy conservation can be applied and used to calculate motion in terms of the
known potential energies of the conservative forces and the work done by nonconservative forces, instead of finding the net work from the net
force, or having to directly apply Newton’s laws.
7.6 Conservation of Energy
• The law of conservation of energy states that the total energy is constant in any process. Energy may change in form or be transferred from one
system to another, but the total remains the same.
• When all forms of energy are considered, conservation of energy is written in equation form as
KEi + PEi + W nc + OEi = KEf + PEf + OEf , where OE is all other forms of energy besides mechanical energy.
• Commonly encountered forms of energy include electric energy, chemical energy, radiant energy, nuclear energy, and thermal energy.
• Energy is often utilized to do work, but it is not possible to convert all the energy of a system to work.
• The efficiency Eff of a machine or human is defined to be Eff = W out
E , where W out is useful work output and E
in
in is the energy
consumed.
7.7 Power
• Power is the rate at which work is done, or in equation form, for the average power P for work W done over a time t , P = W / t .
• The SI unit for power is the watt (W), where 1 W = 1 J/s .
• The power of many devices such as electric motors is also often expressed in horsepower (hp), where 1 hp = 746 W .
7.8 Work, Energy, and Power in Humans
• The human body converts energy stored in food into work, thermal energy, and/or chemical energy that is stored in fatty tissue.
• The rate at which the body uses food energy to sustain life and to do different activities is called the metabolic rate, and the corresponding rate
when at rest is called the basal metabolic rate (BMR)
• The energy included in the basal metabolic rate is divided among various systems in the body, with the largest fraction going to the liver and
spleen, and the brain coming next.
• About 75% of food calories are used to sustain basic body functions included in the basal metabolic rate.
• The energy consumption of people during various activities can be determined by measuring their oxygen use, because the digestive process is
basically one of oxidizing food.
7.9 World Energy Use
• The relative use of different fuels to provide energy has changed over the years, but fuel use is currently dominated by oil, although natural gas
and solar contributions are increasing.
• Although non-renewable sources dominate, some countries meet a sizeable percentage of their electricity needs from renewable resources.
• The United States obtains only about 10% of its energy from renewable sources, mostly hydroelectric power.
• Economic well-being is dependent upon energy use, and in most countries higher standards of living, as measured by GDP (Gross Domestic
Product) per capita, are matched by higher levels of energy consumption per capita.
• Even though, in accordance with the law of conservation of energy, energy can never be created or destroyed, energy that can be used to do
work is always partly converted to less useful forms, such as waste heat to the environment, in all of our uses of energy for practical purposes.
Conceptual Questions
7.1 Work: The Scientific Definition
1. Give an example of something we think of as work in everyday circumstances that is not work in the scientific sense. Is energy transferred or
changed in form in your example? If so, explain how this is accomplished without doing work.
2. Give an example of a situation in which there is a force and a displacement, but the force does no work. Explain why it does no work.
3. Describe a situation in which a force is exerted for a long time but does no work. Explain.
7.2 Kinetic Energy and the Work-Energy Theorem
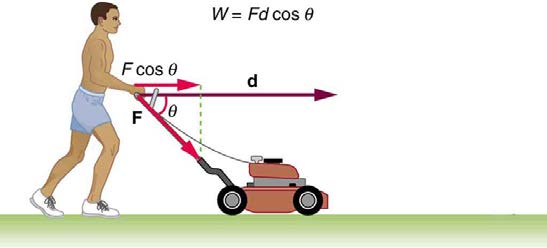
4. The person in Figure 7.33 does work on the lawn mower. Under what conditions would the mower gain energy? Under what conditions would it
lose energy?
CHAPTER 7 | WORK, ENERGY, AND ENERGY RESOURCES 253
Figure 7.33
5. Work done on a system puts energy into it. Work done by a system removes energy from it. Give an example for each statement.
6. When solving for speed in Example 7.4, we kept only the positive root. Why?
7.3 Gravitational Potential Energy
7. In Example 7.7, we calculated the final speed of a roller coaster that descended 20 m in height and had an initial speed of 5 m/s downhill.
Suppose the roller coaster had had an initial speed of 5 m/s uphill instead, and it coasted uphill, stopped, and then rolled back down to a final point
20 m below the start. We would find in that case that it had the same final speed. Explain in terms of conservation of energy.
8. Does the work you do on a book when you lift it onto a shelf depend on the path taken? On the time taken? On the height of the shelf? On the
mass of the book?
7.4 Conservative Forces and Potential Energy
9. What is a conservative force?
10. The force exerted by a diving board is conservative, provided the internal friction is negligible. Assuming friction is negligible, describe changes in
the potential energy of a diving board as a swimmer dives from it, starting just before the swimmer steps on the board until just after his feet leave it.
11. Define mechanical energy. What is the relationship of mechanical energy to nonconservative forces? What happens to mechanical energy if only
conservative forces act?
12. What is the relationship of potential energy to conservative force?
7.6 Conservation of Energy
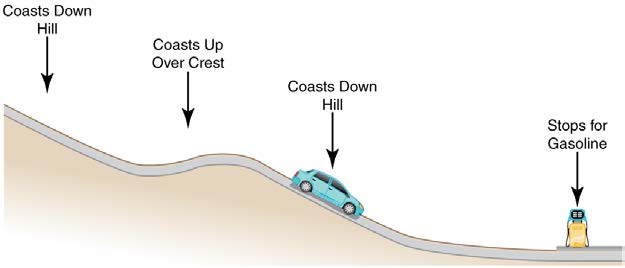
13. Consider the following scenario. A car for which friction is not negligible accelerates from rest down a hill, running out of gasoline after a short
distance. The driver lets the car coast farther down the hill, then up and over a small crest. He then coasts down that hill into a gas station, where he
brakes to a stop and fills the tank with gasoline. Identify the forms of energy the car has, and how they are changed and transferred in this series of
events. (See Figure 7.34.)
Figure 7.34 A car experiencing non-negligible friction coasts down a hill, over a small crest, then downhill again, and comes to a stop at a gas station.
14. Describe the energy transfers and transformations for a javelin, starting from the point at which an athlete picks up the javelin and ending when
the javelin is stuck into the ground after being thrown.
15. Do devices with efficiencies of less than one violate the law of conservation of energy? Explain.
16. List four different forms or types of energy. Give one example of a conversion from each of these forms to another form.
17. List the energy conversions that occur when riding a bicycle.
7.7 Power
18. Most electrical appliances are rated in watts. Does this rating depend on how long the appliance is on? (When off, it is a zero-watt device.)
Explain in terms of the definition of power.
19. Explain, in terms of the definition of power, why energy consumption is sometimes listed in kilowatt-hours rather than joules. What is the
relationship between these two energy units?
20. A spark of static electricity, such as that you might receive from a doorknob on a cold dry day, may carry a few hundred watts of power. Explain
why you are not injured by such a spark.
7.8 Work, Energy, and Power in Humans
254 CHAPTER 7 | WORK, ENERGY, AND ENERGY RESOURCES
21. Explain why it is easier to climb a mountain on a zigzag path rather than one straight up the side. Is your increase in gravitational potential energy
the same in both cases? Is your energy consumption the same in both?
22. Do you do work on the outside world when you rub your hands together to warm them? What is the efficiency of this activity?
23. Shivering is an involuntary response to lowered body temperature. What is the efficiency of the body when shivering, and is this a desirable
value?
24. Discuss the relative effectiveness of dieting and exercise in losing weight, noting that most athletic activities consume food energy at a rate of 400
to 500 W, while a single cup of yogurt can contain 1360 kJ (325 kcal). Specifically, is it likely that exercise alone will be sufficient to lose weight? You
may wish to consider that regular exercise may increase the metabolic rate, whereas protracted dieting may reduce it.
7.9 World Energy Use
25. What is the difference between energy conservation and the law of conservation of energy? Give some examples of each.
26. If the efficiency of a coal-fired electrical generating plant is 35%, then what do we mean when we say that energy is a conserved quantity?
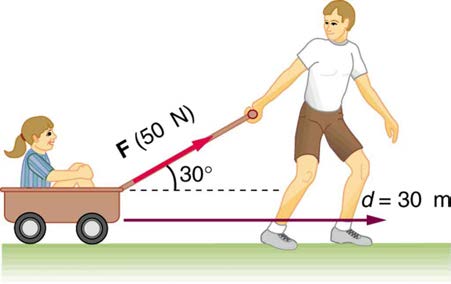
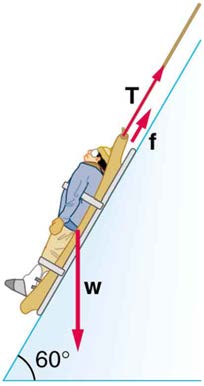
CHAPTER 7 | WORK, ENERGY, AND ENERGY RESOURCES 255
Problems & Exercises









