introduction
At nerdling we’re not afraid to admit that we’re shit-scared of the atomic bomb. We’re also pretty bloody terrified of nuclear waste being mishandled. But these are issues of politics and management, not nuclear science.
I had a discussion about Chernobyl with an academic the other day, where he said, to
substantiate his point that nuclear power facilities are safe, “The system itself was fully under control. It was only because the operators panicked and overrode several safety systems
that there was any sort of problem.” Inadvertently he hit the nail on the head: every good engineering course stresses that people are just as much a part of the system as any nut or bolt or control computer. And until crazy well-intentioned people stop overriding safety systems,
or crazy people stop making more nuclear bombs, or crazy people stop dumping nuclear
waste in places where it shouldn’t go, then we’ll continue to be scared of these things.
However, once the focus moves away from the crazy people and onto the radiation itself,
we’re in a totally different situation. This is where science empowers us.
The role of science in nuclear issues is the same as in any issue—it is not to deny us
the right to moral standpoints and empathic arguments (despite the impression some dead-
ened scientists may give), but rather to allow us to make those arguments stronger by giving
us more information. Becoming a scientist does not mean you cease to be a person, and nor
does it mean you immediately start pissing off your friends and family by turning single-
mindedly pro-nuclear and insisting that they’re all uneducated hippies if they disagree. As
physicist Erwin Schrödinger put it, the ultimate purpose of science is the same as the purpose of the arts—that is, to answer the question “And we, who are we anyway?” Science is about
finding out more about life and the world around us, in order to understand it better. With
more knowledge of a physical system, we need to fear it less.
This is particularly pertinent with respect to nuclear science and radiation—both the ionising radiation dealt with in this zine, and non-ionising radiation such as emitted by mobile
phones—which are perhaps the most feared and misunderstood areas of science relevant to
our daily lives. Because it’s invisible, we have to rely on experts to tell us if it’s really there.
This has elevated the mobile-phone radiation debate to a level where accusations of con-
spiracies and cover-ups are being made. The same sort of vague fear exists about computer
monitors: people I know use radiation filters over their screens ‘just to be sure’. And only a few months ago a boy at a local school was exposed to a caesium radiation source he found
on the road, which resulted in him being rushed to hospital for a full check-up and the story making national news as a ‘radiation scare’—whereas, in reality, you might be able to find a
vase in your grandmother’s house that’s more radioactive than that caesium source (the glaze
on old vases commonly contained uranium due to its pretty honey-brown colour). But this
didn’t stop the story from being a media sensation.
In almost all cases, fear of radiation has done more damage than the radiation itself. Following Chernobyl, thousands of people in Russia were forcibly and permanently moved off their
land due to a fear of contamination—when in many places the radiation levels would, in fact,
have dropped to near-background levels in only a few days. The suicides resulting from the
trauma of losing all their land and heritage took a greater toll on the population than the ra-2

diation would have caused. And when the European public heard about the plume from
Chernobyl that was carried over the continent, abortions of foetuses skyrocketed—again,
unnecessarily.
Remember, radiation itself is not intrinsically bad. It’s all around us; right now there is radiation from the earth’s crust and from the sun zipping through your body all over the place, not doing you any harm. Your body itself contains Carbon-14 and Potassium-40, which are radioactive particles zapping away at your DNA non-stop—which is why our body has devel-
oped mechanisms to deal with most DNA damage from radiation before it goes on to form a
cancer. It’s the crazy people that are the problem—but that’s the case with everything. Re-
member, the fact that a bloody big asteroid could come and wipe most of the life on earth
doesn’t mean we have to be afraid of rocks in general. So too with radiation.
This zine does not even try to be a comprehensive guide to radiation. That’s what textbooks
are for. We’ve thrown in just enough science to give you a feel for what’s going on, and then it’s up to you to find out more if you’re interested. There are some good references cited
along the way for further reading.
Sit back, strap on your weird radiation-proof goggles, and enjoy the zine.
the übernerdling
editor, nerdling zine
january 2004
email: editor@nerdling.net
web: www.nerdling.net
3
contents
The Basics
p 2: Introduction
p 6: Nuclear Physics 101
The culprit is identified.
Radiation
p 8 : Introduction to Measuring Radiation
Subtitled, “units suck”
p 9: Marie Curie: Radiation Pioneer, Total Babe
p 10: Radiation Cures
“Real, healthful radioactive water . . . nature's way to health.”
p14: Nuclear Free, Schmuclear Free
Think your town is ‘nuclear free’? Think your own body is ‘nuclear
free’? What about those bananas there? You might be in for a surprise.
p 17: Calculate Your Own Radiation Dose
Use Colin Keay’s nifty table to add up how much radiation you get
zapped by every year.
p 18: Radiation Treasure Hunt
Discover radioactive vases, lemon juicers, plant pots, and lamp stands
that light up the room even when unplugged.
p 21: Great Uses for Radioactive Materials #1: Make Some Cash on E-Bay
The Bomb
p 22: Nuclear Fusion & Nuclear Fission
E=mc2, or how those little dudes pack so much punch
p 24: How to Build a Nuclear Bomb
A rough guide to the A-bomb, H-bomb and neutron bomb.
p 25: Great Uses for Nuclear Weapons #2: Fight Hurricanes
4
p 26: The Trinity Test
“I am become Death, the Destroyer of Worlds.”
p 28: The Nevada Testing Site
Pigs in uniforms, dogs in cages, and a fun family holiday destination
p 30: Nuclear Testing in Australia
Yes, weapons can hurt people and contaminate land during peacetime too.
p 33: Fun Nuclear Facts
p 34: Differing Accounts of Nuclear Explosions
“To every man is given the key to the gates of heaven; the same key opens
the gates of hell.”
p 38: “My Greatest Thrill”: the Bombing of Nagasaki
p 39: Great Uses for Old Nuclear Weapons #3: Go To Mars
p 40: Atomic Era Pop Culture featuring the Bikini
It’s all fun and games...
p 44: Atomic Era Paranoia
… until someone loses an eye.
p 46: The Superheroes’ Guide on What To Do In Case Of Nuclear Attack
p 48: Great Uses for Old Nuclear Weapons #4: Dig A Canal, Build A Harbour
The Reactor
p 50: Lucas Heights: Australia’s Only Nuclear Facility
p 52: Build Your Own Breeder Reactor
Forget those IKEA bookshelves and try some real DIY.
p 55: Westinghouse and Nuclear Power
p 56: Nuclear Waste
Love it or hate it. You decide.
p 58: Nuclear Fusion: the Beginner’s Guide
5
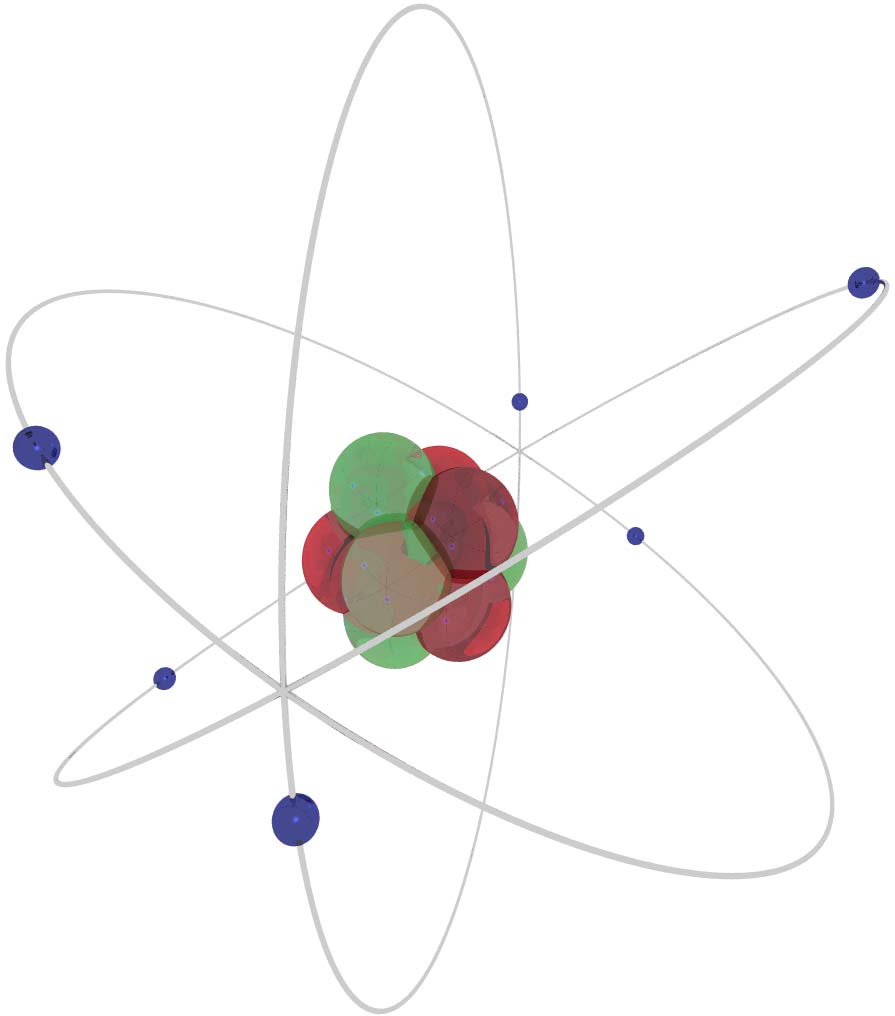

EVERYTHING IN
THIS ISSUE
INVOLVES THIS
THE ATOMIC
NUCLEUS
6
• The atomic nucleus consists of protons
and neutrons (called nucleons) tightly packed to-
gether. Protons have a positive charge while neutrons have
no electrical charge. Both weigh about 1.7×10-27 kg; in
other words it would take about a thousand
million million million million of them to
make up a gram.
• The number of protons determines which chemical element
the atom is. There are usually at least the same number of
neutrons present, and sometimes more. Atoms of the
same element, but with different num-
bers of neutrons, are called isotopes (from
the Greek isos meaning ‘same’ and topos for ‘place’ - hav-
ing the same place in the periodic table).
• Protons and neutrons are about 2 femtometers wide (1fm =
10-15m). If you know A, the total number of nucleons, you
can find the nuclear radius in femtometers by the formula
R=1.2×A1/3. Using this, we can find that a carbon nucleus is
about 2.7 fm wide. The width of the whole car-
bon atom, by comparison, is about
30,000 times greater. You can see that the pic-
ture to the left is hardly drawn to scale.
• The nucleons are held together by the Strong Nuclear
Force. It only acts over very short distances of about 1 fm,
but is the strongest force known: between two neighbour-
ing protons, the strong nuclear force is
about 100 times greater than the elec-
trostatic force and roughly 1034 times
stronger than the gravitational force.
• It looks fairly innocent. Don’t let this fool you.
7
For those of us who aren’t exactly Marie Curies, we’ve condensed
most of the boring science in this zine to just one page. If you’re in-
terested in an introduction to radiation science and measurement,
get stuck into the print below. Otherwise, skip over a few pages to
where there’s some pretty wacky stuff and a few cartoon charac-
ters and big explosions to keep you entertained.
Ionising radiation, the stuff we’re concerned with in this zine, is
nothing but little particles zapping out at you. Mostly these particles
are spat out of heavy elements like uranium, or unstable isotopes like
carbon-14, both of which would rather exist as something else.
There are many particles which can be considered ‘radiation’, but
most often we’re concerned with alpha particles (pretty large, made of two
protons and two neutrons), beta particles (a fancy name for flying elec-
dioactivity trons), gamma rays (just high-energy light), x-rays (more high-energy light)
and neutrons.
Radiation can be dangerous to living organisms because it can knock bits
out of important molecules. Contrary to popular belief, our bodies are getting
zapped with natural radiation all the time. This radiation can occasionally mess
with our DNA, but our body is actually pretty good at repairing this damage on
the level of day-to-day exposure.
How to measure radiation? Here’s where researching radiation can be like
trying to navigate through a quagmire using a map written randomly in seven different
languages.
There are seven different units for measuring radiation, summarised in the table
below, and converting between them is not as easy as you might think. So why is measuring
ide to ra radiation not as straightforward as measuring length or time? Well, unlike length or time we’re not only interested in the physical properties of the radioactive substance, but also
its effect on humans. This effect varies with the type of radiation, the exposure amount,
and the exposure time.
A Geiger counter can register each time one of these particles passes through a given
space. This gives a reading in Bequerels (or Curies). However, this measurement will not only vary with the distance from the source but also the size of the detector. It also gives no information about how damaging the particles are to tissue.
The unit Gray takes the energy of the particles into account, and the Sievert takes the energy and the level of tissue damage into account. To calculate the dose in Sieverts, one takes the dose in Grays and multiplies it by a ‘quality factor’ (QF) corresponding to the particles’ ability to damage tissue. This factor is exactly 1 for x-rays, approximately 1 for gamma and beta rays, 2-5 for thermal neutrons, about 10 for fast neutrons and protons, and 10-20 for heavy charged particles such as alphas.
As an example, say I want to compare the radioactivity of a barrel of nuclear waste (15 millirem) to the activity of the uranium dye in an old vase I own, which registers 1500 counts per
minute on a Geiger counter. To convert the units
Table 1: Units Suck
from counts per minute to millirem is not as easy
as you might think. There are actual y at least
Name
Standard Unit
Old Unit
seven different units commonly used to measure
e-page gu radiation. In this case we’d first have to convert
the 15 millirem (old unit, like the mile) to Sieverts
Activity
Becquerel (Bq)
Curie (Ci)
(new unit, like the kilometre). Then we’d have to find
of Source = 1 disintegration/s = 3.7×1010 Bq
out exactly what kind of radiation the waste was
giving out (less easy than you think) and use this to
convert to Grays (radiation energy per kilogram of
your body), and then to Bequerels (counts per
Absorbed Gray (Gy)
rad
minute). Then you’d have to analyse the type of
Dose
= 1 J/kg
= 10-2 Gy
radiation given off by my uranium vase to find out the
Roentgen
proportion of alpha particles, which aren’t going to be
picked up by the detector (they can’t penetrate the
= 8.7 mGy
casing) and hence need to be mathematically compen-
sated for. By this time the numbers can be compared,
Dose
Sievert (Sv)
Rem
but are probably so ful of guestimates that it’s hardly
meaningful anyway.
Equiva-
=(dose in Gy)×QF
= 10-2 Sv
the on
lent
If you can read this, you’re way too enthusiastic.
8























Marie Curie: Radiation Pioneer, Total Babe.
Not only did she win two Nobel prizes for her pioneering work on radioactivity, risk her life to build and bring mobile x-ray units to the front lines of the war, and establish the use of radiation in medicine at the expense of her own life, but she lived modestly and always true to her beliefs. A true modern heroine.
9

Radioactive Jockstraps, Suppositories and Tablets:
Cure Yourself Through Radiation!
Nowadays most people have an acute fear of radiation. Mention to a friend that bananas
have quite a high level of natural radioactive potassium and it’s quite possible that they
will think twice before having another banana split. So it can be quite astounding to dis-
cover that as recently as the 1980s people were using commercially available radioactive
products in the belief it would bring good health.
The idea goes back to the ancient notion that certain springs have healing proper-
ties. For thousands of years, people have journeyed to places like Bath in England and
Badgastein in Austria seeking cures in the waters there.
When it was discovered around 1903 that the water in most of these springs was
slightly radioactive (due to the presence of radon gas in the ground through which the
water flows), the world leapt to the conclusion that radiation was the magical healing
agent in the springs.
The American Journal of Clinical Medicine published the statement that
“Radioactivity prevents insanity, rouses noble emotions, retards old age, and creates a
splendid youthful joyous life.” According to Professor Bertram Boltwood of Yale, radia-
tion cures worked by “carrying elec-
trical energy into the depths of the
The Revigator (1920):
body and there subjecting the juices,
Irradiate Your Water
protoplasm, and nuclei of the cells to
“Results overcome doubts. …
an immediate bombardment by ex-
The millions of tiny rays that
plosions of electrical atoms” which
are continuously given off by
was, amongst other things, “an agent
this ore penetrate the water and
for the destruction of bacteria”.
form this great HEALTH ELE-
Radon was believed to be the
MENT—RADIO-ACTIVITY. All
‘life element’ of water. Radon was
the next day the family is pro-
to water what oxygen was to air.
vided with two gallons of real, healthful radioac-
Naturally, entrepreneurs took note—
tive water . . . nature's way to health.”
radioactivity was going to be big
business.
The problem for people seeking radioactive cures was that radon does not stay in
water for very long. After a short period, it decays or escapes into the air, meaning it has
to be drunk (or bathed in) at the source. An invention in 1912 aimed to overcome this
problem and bring the miracle healing properties of radiation to people everywhere.
Called the Revigator, it was a “radioactive water crock” made of radium-containing ore
which could hold several gallons of water. The instructions on the side read: “Fill jar
every night. Drink freely … when thirsty and upon arising and retiring, average six or
more glasses daily.” The Revigator Company, based in San Francisco, sold several hun-
dred thousand of these products, which they called “a perpetual health spring in the
home.”
10



Many copy-cat products quickly followed. The American Medical Association
(AMA) was naturally concerned that the public was being fleeced by charlatans. To pre-
vent this they established guidelines (in effect from 1916 to 1929) for a minimum radiation level that was acceptable from these devices. If it was putting out less than 2 µCi of radon per litre of water in 24 hours, then you were being fleeced! Even the famous Revigator was not radioactive
enough to meet these standards.
The Radiendocrinator (1930):
In Australia, too, certain mineral
Irradiate Your Scrotum
waters were guaranteed by gov-
ernment authorities to be radio-
“Place Radiendocrinator in the
active.
adapter . . . wear like any ath-
By the 1920s and 30s, it
letic strap . . . under the scrotum
as it should be. Wear at night.
was thought that eating radioac-
Radiate as directed.”
tive compounds, or rubbing
them directly on to you, could
be even more effective than drinking the water. Thus were born radium-containing cos-
metics, ear plugs, bath salts, soap, and even suppositories. You could eat a bar of radio-
active chocolate, wash it down with a German radioactive beer,
and then brush your teeth with radioactive toothpaste. Radium
blankets were available to keep you warm at night, and women
could wear radium-corsets during the day. Men used radiation
treatments to enhance virility, and women used radiation contra-
ceptives in case the mens’ treatment unexpectedly worked.
The Radiendocrinator (pronounced Ra-di- en-do-cri-na-
tor), for example, consisted of refined radium encased in 14-carat
gold and shipped in a velvet-lined leatherette case for $150—and
was designed to be strapped to the scrotum, where it would
stimulate the endocrine glands “which have so masterful a con-
trol over life and bodily health.” Also on the market, for only $1
per box of 42, were Radium tablets designed to be taken with
each meal. Then there was the Radium Respirator, which en-
Radithor (1928)
abled you to breath ‘radon-purified’ air. To quote the manufac-
“Harmless in every
turer: “Radium: scientists found it, governments approved it,
respect.”
physicians recommended it, users endorse it, we guarantee it,
SURELY IT’S GOOD!”
Radithor was triple-distilled water
guaranteed to contain at least 1 microcurie
The Radium Emanation Bath (1925)
of Radium. Its inventor was William J.
Bailey, the same man who brought the
Described as being good for nervous disor-
world the Radiendocrinator. He proudly
ders, insomnia, general debility, arthritis, and
rheumatism. “Empty con-
used all of his products and claimed to
tents in a quart of hot water.
have drunk more radium water than any
After a few moments add to
living man. He died in 1949 of bladder
regular bath solution. Re-
cancer at 64 years of age.
main in bath 45 minutes
Radithor was advertised as being
with cover over top of tub.
‘Harmless in every respect.” The product
Upon leaving bath relax in
slogan proved false in the case of Eben

































