1. Symmetry Elements
Using the Molecular Framework models, make models of the following compounds:
1. CH4
2. CH3Cl
3. CH2Cl2
4. CHCl3
5. CH2ClF
6. CHBrClF
7. BF3
8. BF2Cl
9. PH3
10. PH2Cl
Choose a color to represent each atom. For example, make all C atoms black, all H atoms white,
etc.
Once the models are created, look for symmetry elements that may be present. Ask yourselves the following questions:
Does the molecule contain a mirror plane (σ)? In other words, is there a plane within the molecule which results in one half being a mirror image of the other half?
Does the molecule contain a two-fold rotation axis (C2)? Remember from the Introduction
that the subscript indicates the degrees of rotation necessary to reach a configuration that is
indistinguishable from the original one. In this case, the rotation will be 180o.
Does the molecule contain any higher-order rotation axes?
C3 – rotation by 120o
C4 – rotation by 90o
C∞ (infinity rotation axis) – rotation of any amount will result in an indistinguishable
orientation
Does the molecule have an inversion center (i)?
Determine which of these symmetry elements are present in your assigned molecules. All of the
columns of the table on the report form should be filled out. If you have any difficulty
determining whether such symmetry elements are present in the molecules you are assigned, your
TA can provide examples of each symmetry element.
Extra credit points can be earned by indicating in the table how many of each symmetry element
are present for each molecule (i.e. How many mirror planes are present?).
2.Mirror Images
Using the model kits, build models which are the mirror images of the models you were assigned
to build (b, c, d, e, f, g, h, i and j) in Part 1. With the two mirror images in hand, try to place the models on top of one another, atom for atom.
If you can do this, the model and its mirror image are superimposable mirror images of one another. If not, the molecule and its mirror image form nonsuperimposable mirror images.
Nonsuperimposable mirror images are also known as enantiomers.
For each compound, decide whether the mirror image is superimposable or nonsuperimposable.
Can you make a generalization about when to expect molecules to have nonsuperimposable mirror
images?
3.Isomers
In this exercise you will build models of compounds which are structural and/or geometrical
isomers of one another.
Make the following models:
A. Structural Isomers
1. Make a model(s) of C2H5Cl. How many different structural isomers are possible?
2. Make a model(s) of C3H7Cl. How many different structural isomers are possible?
3. Make a model(s) of C3H6Cl2. How many different structural isomers are possible?
B. Geometrical Isomers
1. Make a model(s) of C2H3Cl. How many different structural and geometrical isomers are
possible?
2. Make a model(s) of C2H2Cl2. How many different structural and geometrical isomers are
possible?
3. Make a model(s) of cyclobutane (C4H8). HINT: cyclo = ring of C atoms
4. Now make dichlorocyclobutane (C4H6Cl2) by replacing two H atoms on cyclopropane with Cl
atoms. How many different structural and geometrical isomers are possible for
dichlorocyclobutane? You may wish to make a couple of cyclobutane molecules so that you
can compare the structures. Do any of the isomers have nonsuperimposable mirror images?
C. Aromatic Ring Compounds
1. Make a model of benzene, C6H6. Even though your model will contain alternating double and
single bonds, remember that in the real molecules of benzene all the C-C bonds are equivalent.
What symmetry elements does benzene possess?
2. Make a model(s) of chlorobenzene, C6H5Cl. How many different structural and geometrical
isomers are possible?
3. Make a model(s) of dichlorobenzene, C6H4Cl2. How many different structural and geometrical
isomers are possible?
4. Make a model(s) of trichlorobenzene, C6H3Cl3. How many different structural and geometrical
isomers are possible?
4. Hypervalent Structures
Hypervalent compounds are those that have more than an octet of electrons around them. Such compounds are formed commonly with the heavier main group elements such as Si, Ge, Sn, Pb, P,
As, Sb, Bi, S, Se, Te, etc. but rarely with C, N or O. Refer to the rules for Electron Domain theory in order to assign Lewis structures to the following molecules. Describe possible isomeric forms and the bond angles between the atoms. How many lone pairs of electrons are present on the
central atom of each molecule, if any? (** Your book will be very useful in aiding you with these structures **)
1. PF5
2. PF3Cl2
3. SF4
4. XeF2
5. BrF3
5. BrF3
Solutions
Chapter 6. Nanotechnology: Ferrofluids and Liquid
Crystals
Nanotechnology: Ferrofluids and Liquid Crystals
Objective
To synthesize an aqueous ferrofluid (magnetite) and observe its properties.
To understand how nanotechnology affects everyday life.
To learn about surfactants and how they work.
Grading
Pre-Lab (10%)
Lab Report Form (80%)
TA Points (10%)
Background Information
Nanotechnology is the science of controlling matter with dimensions between 1 and 100
nanometers. This includes manipulating individual molecules. It is a multidisciplinary field
consisting of physics, biology, chemistry, medicine, engineering, and nearly any other applied
science. The prefix nano- means ten to the minus ninth power, or one billionth. There have been
great advances in nanotechnology in recent years, and scientists routinely make materials that are only a few nanometers in size, or about 1/80,000 the diameter of a human hair. See Figure 1 to
notice how small a nanometer is compared to other common materials.
Materials at the nanoscale exhibit interesting optical, electronic, physical, and chemical properties due to their small size. For example, catalysis chemical reactions occur at the surface of bulk
material so as particles become smaller, the ratio of the surface area to the volume of the particles increases, thereby making a volume of nanoparticle catalysts more reactive than an equal volume
of bulk catalyst. Optical properties of bulk materials are not size dependant, that is no matter what the size of a piece of bulk material it will have the same optical properties. This is not the case for nanomaterials. As you will see in the instructor demo, different sizes of gold nanoparticles exhibit very different colors.
In the 1960s NASA Research Centers discovered fluids that could be controlled through the
application of a magnetic field. These fluids were developed to confine liquids in space. These
nanoparticle fluids are commonly known as ferrofluids and they are still an active area of
research.
Ferrofluids have many current industrial applications. They are used to dampen vibration in audio loudspeakers, they can behave as liquid O-rings in rotating shaft seals, and they are used in high-speed computer disk drives to eliminate impurities. They also have many potential applications in biomedical, environmental, and engineering fields.
Figure 6.1.
Figure 1-Obtained from Office of Basic Energy Sciences, US Department of Energy
A ferrofluid is a stable colloid suspension of magnetic nanoparticles in a liquid carrier. The
nanoparticles are suspended throughout the liquid and have an average size of ~10 nm. It is
critical that the nanoparticles are coated with surfactant to prevent the particles from aggregating together. The surfactants must be strong enough to prevent agglomeration even when a magnetic
field is applied and they must overcome the intermolecular forces between the nanoparticles. For this reason, a typical ferrofluid contains 5% magnetic nanoparticles, 10% surfactant, and 85%
carrier fluid by volume.
Figure 6.2.







There are two basic steps in creating a ferrofluid: synthesis of the magnetic solid, magnetite (
), and suspension in water with the aid of a surfactant. The magnetic particles must be very
small on the order of 10 nm (100 Å) in diameter, so that the thermal energy of the particles is
large enough to overcome the magnetic interactions between particles. If the particles are too
large, magnetic interactions will dominate and the particles will agglomerate. The magnetite will be synthesized by a precipitation reaction that occurs upon mixing
and
with ammonium
hydroxide (an aqueous solution of ammonia,
). The unbalanced equation for this reaction is as
follows:
(6.1)
The surfactant used in this synthesis is tetramethylammonium hydroxide (
). The
hydroxide (
) ions formed in solution tend to bind to the iron sites on the magnetite particles,
creating a net negative charge on each particle. The positively-charged tetramethylammonium
ions will then associate with the negatively-charged magnetite particles, forming a kind of shell around each magnetite particle. This charged shell raises the energy required for the particles to agglomerate, stabilizing the suspension.
Changing the subject to liquid crystals: with the help of nanotechnology, liquid crystal displays have become very popular in recent years. Liquid crystal displays (LCD) were first produced by
RCA in 1971 and are composed of two glass plates with a liquid crystal material between them.
The liquid crystal material is an organic compound that is in a state between a liquid and a solid.
Their viscosities are similar to those of liquids and their light scattering and reflection properties are similar to solid crystals. Liquid crystals must be geometrically highly
anisotropic (having different optical properties in different directions)-usually long and narrow -
but also become an isotropic liquid (same optical properties in all directions) through a stimulus such as a magnetic field, electric field, temperature, or pressure.
Liquid crystals have several common phases. The simplest liquid crystal phase is called the
nematic phase where the molecules spontaneously order with long axes roughly parallel. It is
characterized by a high degree of long range orientational order but no translational order. An
uniformly aligned nematic has a preferred direction, often described in terms of a unit vector
called the director. The type of phase that a liquid crystal possesses ultimately determines its applications.
A subclass of nematic phases that will be investigated in this lab due to its pressure and
temperature sensitive properties is the cholestric phase. The distance over which the director
rotates to equal 360° is referred to as the chiral pitch and is normally on the order of a few
hundred nanometers, or precisely the wavelength of visible light. This allows liquid crystals to selectively reflect light of wavelengths equal to the pitch length, so that a color will be reflected when the pitch is equal to the corresponding wavelength of light in the visible spectrum. Changes in the director orientation between successive layers modifies the pitch length resulting in an
alteration of the wavelength of reflected light according to the temperature. The angle at which the
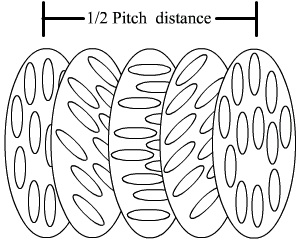
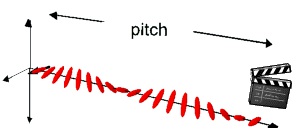


director changes can be made larger, and thus tighten the pitch, by increasing the temperature of the molecules, hence giving them more thermal energy. Similarly, decreasing the temperature of
the molecules increases the pitch length of the chiral nematic liquid crystal. This makes it
possible to build a liquid crystal thermometer that displays the temperature of its environment by the reflected color.
Figure 6.3.
Figure 6.4.
Experimental Synthesis
Part I. Synthesis of Gold Nanoparticles (DEMO)
Chemicals
1.0 mM
(stored in amber bottle)
1% trisodium citrate dihydrate solution
Nanoparticle Synthesis
Add 20 mL of 1.0 mM
to a 50 mL Erlenmeyer flask on a stirring hot plate. Add a
magnetic stir bar and bring the solution to a boil.
Add 2 mL of 1% trisodium citrate dihydrate solution and observe the properties.








Part II. Synthesis of the Aqueous Ferrofluid (Procedure modified from J. Chem.
Edu. 1999, 76, 943-948.)
Chemicals
1 M
in 2 M HCl Solution:
2 M
in 2 M HCl Solution
25% tetramethylammonium hydroxide in water
1.0 M
Solution: Dilute at least 200 mL of concentrated ammonium hydroxide with water to
3.0 L). Open containers of ammonia are odorous and their concentration will decrease over
periods of time.
CAUTION: Ferrofluids can be messy. This particular ferrofluid will permanently stain almost all
fabrics. Also DO NOT LET THE MAGNETITES TOUCH THE SURFACE OF THE MAGNET
DIRECTLY.
Magnetite Synthesis
In a hood, place 4.0 mL of 1M
and 1.0 mL of 2M
solution into a 100 mL beaker. Stir
on a magnetic stir plate.
While stirring, slowly add 50 mL of 1.0 M aqueous
solution over a 5 minute period using a
buret. Initially a brown precipitate will form followed by a black precipitate, which is
magnetite.
CAUTION: Even though 1M
is fairly dilute,
is a strong base.
Remove from stirring and immediately use a strong magnet to work the stir bar up the walls of
the beaker. Remove the stir bar with a gloved hand being careful not to let it touch the magnet.
Allow the magnetite to settle, then decant off the clear liquid into a waste beaker without losing a large amount of precipitate. The settling process can be expedited by placing a strong magnet
below the beaker.
Transfer the solid to a plastic weighing boat. Rinse out the beaker with a wash bottle.
Use a strong magnet to attract the ferrofluid to the bottom of the weigh boat. Carefully decant
as much clear liquid as possible into the waste beaker. Rinse again with water from the wash
bottle and decant. Repeat the rinsing process a third time. What are you removing by rinsing?
Add 1-2 mL of 25% tetramethylammonium hydroxide. Gently stir the solution with a glass stir
Add 1-2 mL of 25% tetramethylammonium hydroxide. Gently stir the solution with a glass stir
rod for at least a minute to suspend the solid in the liquid. Use a strong magnet to attract the ferrofluid to the bottom of the weigh boat. Pour off and discard the dark liquid. Move the strong magnet around and again pour off any liquid. If the ferrofluid does not produce spikes, continue to move the strong magnet around, pouring off any liquid.
Ferrrofluid Properties
1. Hold a magnet underneath the weigh boat that contains the ferrofluid. Move the magnet
around the underside of the weigh boat. Move the magnet close to and far from the weigh boat.
Record your observations
2. Add a couple of drops of ferrofluid to a small piece of clean paper. Let the solution dry. Once it is dry, bring a strong magnet close to the paper. What happens?
3. Use several different magnets to observe the properties of the ferrofluid and record your
observations in your notebook.
Part III. Synthesis of Cholesteryl Ester Liquid Crystals
Chemicals
Cholesteryl oleyl carbonate
Cholesteryl pelargonate
Cholesteryl benzoate
Vials
Heat gun
Liquid Crystal Synthesis
1. Place 0.65 g cholesteryl oleyl carbonate, 0.25 g cholesteryl pelargonate, and 0.10 g cholesteryl benzoate in a vial.
2. In a hood, with the cap off, melt the solid in with a heat gun.
3. While the mixture is still a liquid, divide it into separate vials using a disposable pipette. Put the caps back on the vials. Allow the vials to cool and observe their properties.
4. Clean up your bench area.
5. Listed below is a chart of the different ratios that produce liquid crystals with different
5. Listed below is a chart of the different ratios that produce liquid crystals with different
transition temperatures. Placing liquid crystals with different transition temperatures next to
each other on a clear piece of contact paper makes it possible to make a thermometer.
Table 6.1.
Cholesteryl oleyl
Cholesteryl
Cholesteryl
Transition range,
carbonate (g)
pelargonate (g)
benzoate (g)
degrees C
0.65
0.25
0.10
17-23
0.70
0.10
0.20
20-25
0.45
0.45
0.10
26.5-30.5
0.43
0.47
0.10
29-32
0.44
0.46
0.10
30-33
0.42
0.48
0.10
31-34
0.40
0.50
0.10
32-35
0.38
0.52
0.10
33-36
0.36
0.54
0.10
34-37
0.34
0.56
0.10
35-38
0.32
0.58
0.10
36-39
0.30
0.60
0.10
37-40
Solutions

Chapter 7. Solid State and Superconductors
Solid State Structures and Superconductors
Objectives
Build examples of: simple cubic, body centered cubic and face centered cubic cells.
Understand and familiarize with three-dimensionality of solid state structures.
Understand how binary ionic compounds (compounds made up of two different types of ions)
pack in a crystal lattice.
Observe the special electromagnetic characteristics of superconducting materials using 1,2,3-
superconductor
, discovered in 1986 by Dr. Paul Chu at the University of Houston.
Grading
Your grade will be determined according to the following
Pre-lab (10%)
Lab report form. (80%)
TA points (10%)
Before coming to lab:
Read introduction and model kits section
Complete prelab exercise
Introduction
From the three states of matter, the solid state is the one in which matter is highly condensed. In the solid state, when atoms, molecules or ions pack in a regular arrangement which can be
repeated "infinitely" in three dimensions, a crystal is formed. A crystalline solid, therefore, possesses long-range order; its atoms, molecules, or ions occupy regular positions which repeat in three dimensions. On the other hand an amorphous solid does not possess any long-range order.
Glass is an example of an amorphous solid. And even though amorphous solids have very


interesting properties in their own right that differ from those of crystalline materials, we will not consider their structures in this laboratory exercise.
The simplest example of a crystal is table salt, or as we chemists know it, sodium chloride (NaCl).
A crystal of sodium chloride is composed of sodium cations (
) and chlorine anions (
) that
are arranged in a specific order and extend in three dimensions. The ions pack in a way that
maximizes space and provides the right coordination for each atom (ion). Crystals are three
dimensional, and in theory, the perfect crystal would be infinite. Therefore instead of having a molecular formula, crystals have an empirical formula based on stoichiometry. Crystalline
structures are defined by a unit cell which is the smallest unit that contains the stoichiometry and the “spatial arrangement” of the whole crystal. Therefore a unit cell can be seen as the building block of a crystal.
The crystal lattice
In a crystal, the network of atoms, molecules, or ions is known as a crystal lattice or simply as a lattice. In reality, no crystal extends infinitely in three dimensions and the structure (and also properties) of the solid will vary at the surface (boundaries) of the crystal. However, the number of atoms located at the surface of a crystal is very small compared to the number of atoms in the interior of the crystal, and so, to a first approximation, we can ignore the variations at the surface for much of our discussion of crystals. Any location in a crystal lattice is known as a lattice point.
Since the crystal lattice repeats in three dimensions, there will be an entire set of lattice points which are identical. That means that if you were able to make yourself small enough and stand at any such lattice point in the crystal lattice, you would not be able to tell which lattice point of the set you were at – the environment of a lattice point is identical to each correspondent lattice point throughout the crystal. Of course, you could move to a different site (a non-correspondent lattice point) which would look different. This would constitute a different lattice point. For example, when we examine the sodium chloride lattice later, you will notice that the environment of each
sodium ion is identical. If you were to stand at any sodium ion and look around, you would see the same thing. If you stood at a chloride ion, you would see a different environment but that
environment would be the same at every chloride ion. Thus, the sodium ion locations form one set of lattice points and the chloride ion locations form another set. However, lattice points not only exist in atom positions. We could easily define a set of lattice points at the midpoints between the sodium and chloride ions in the crystal lattice of sodium chloride.
The unit cell
Since the crystal lattice is made up of a regular arrangement which repeats in three dimensions, we can save ourselves a great deal of work by considering the simple repeating unit rather than the entire crystal lattice. The basic repeating unit is known as the unit cell. Crystalline solids often have flat, well-defined faces that make definite angles with their neighbors and break cleanly
when struck. These faces lie along well-defined directions in the unit cell.
The unit cell is the smallest, most symmetrical repeating unit that, when translated in three
dimensions, will generate the entire crystal lattice.
It is possible to have a number of different choices for the unit cell. By convention, the unit cell that reflects the highest symmetry of the lattice is the one that is chosen. A unit cell may be
thought of as being like a brick which is used to build a building (a crystal). Many bricks are
stacked together to create the entire structure. Be


























