efficient compaction techniques, particularly with silts,
For these latter types of structures it is also very
clays, and mixed soils.
important that design densities not be exceeded.
Excessive compaction might result in undesirable lateral
The two most common standards for
stresses in structural members.
compaction control are the standard Procter (ASTM D-
698-58T) [6-7] and the modified Procter (ASTM D-1557-
6-2
6.2.3 TYPES OF COMPACTION. The type of
thickness of no more than 6 inches, except in cases
compaction selected should be based upon the soil type.
where it has been demonstrated that the compactors are
Vibratory compaction is most efficient with granular
capable of achieving the required densities throughout
soils, whereas kneading types of equipment, such as
the full depth of thicker lifts. Such a situation might
sheepsfoot rollers, are more applicable to cohesive
exist with a vibratory compactor on relatively clean,
soils. There is no ready means of controlling the
cohesionless material. For backfilling sheet pile
compaction of coarse rock fill. Vibratory rubber-tired
cofferdams or other structures of limited extent, it may
compactors in the 12-to-15-ton range are reasonably
be necessary to use small hand-operated tampers or
effective for all types of soil. Soil lift thicknesses should
compactors. The importance of compaction generally
generally be limited to soil layers having a compacted
increases with decrease in grain size.
SECTION 3. INSPECTION
Inspection of waterfront soil structures is
level. In cases where damage is noted or suspected
generally limited to visual observations. In some cases
(such as the observance of escaping backfill at the toe
piazometers, survey stations, or other devices may be
of a wall, the presence of sediment in the seepage
installed into the structure to facilitate inspection.
water, or surface slumping), other techniques, such as
Unfortunately, the corrosive nature of the waterfront
drilling exploratory boreholes or using sonic or
environment inhibits the long-term performance of most
geophysical techniques, should be resorted to (see
permanently installed instrumentation. Pressure-
Reference 1-10, Chapter 2). A number of sophisticated
relieved structures, such as some drydocks, are
nondestructive testing techniques, such as those using
susceptible to destructive uplift pressures due to failure
reflected electromagnetic waves, are occasionally
of the dewatering system, development of erosion
suggested, but the nature of the waterfront, particularly
channels, or deterioration of flow cut-offs, etc. Regular
the salty ocean environment, drastically impairs the
monitoring of pore pressure levels beneath the structure
value of such methods.
must be carried out and reference made to the design
SECTION 4. REPAIR
6.4.1 EROSION. The major maintenance requirement
sufficient riprap or slope protection on side slopes or
for earth-filled waterfront structures consists of
insuring the integrity of soil-containing structures, such
preventing the soil from being eroded and replacing the
as sheet pile cofferdams, timber or concrete paneled
soil when a loss is discovered. This means maintaining
quaywalls, etc. In cases where there is evidence of
6-3
erosion or loss of soil, any protective covering, such as
barriers, such as sheet piles. Where soil permeabilities
rockfill or armor units, should be removed, and the
are greater than 1 x 10-3 fpm, subsurface drainage by
internal fill material inspected. Any necessary repairs in
well points, deep wells, etc., may be necessary (see
the form of replacement of properly compacted soil
Reference 1-10, Chapter 8). Prior to planning
should be made, and the protective slope covering
dewatering procedures it is necessary to determine
replaced in a manner to insure no further erosion. This
permeability and piezometric levels by field
may require a series of soil layers of varying coarseness
observations (see Reference 1-10, Chapter 4). The
to insure that the finer, central materials cannot be
major criterion is to avoid instability through piping or
washed out through the coarser, shell materials. In
heaving. This is achieved by controlling the upward
some cases it may be desirable to protect the side
hydraulic gradient at the base of the excavation.
slopes of the structure by such means as asphaltic
Hydraulic gradients (head loss per unit length of
concrete, soil cement, or even reinforced portland
flowpath) of one lead to immediate instability in all
cement concrete. In cases where the side slopes are
cases, but exit gradients of 0.5 to 0.75 will cause
exposed only to atmospheric erosion, vegetation such
unstable working conditions even in clean sands. Silty
as ice plant, grasses, etc., might be adequate.
materials are even more critical.
6.4.2 SEALING. The loss of soil from behind quaywalls
6.4.4 GROUTING. In some cases it may be more
or from within sheet pile cofferdams, etc., requires
expedient to either seal or repair a damaged structure
sealing of the structure to prevent further loss of
by injecting grout (see Reference 1-10, Chapter 15, or
material and replacement of suitable backfill.
Reference 6-9). Grout may be used to reduce the
Coarsergrained materials are generally preferred where
permeability of the soil fill or its foundation and, thereby,
they are available, since they are less subject to
minimize erosion or leaching. It may also be used to
leaching or erosion. Fine-grained materials are
physically strengthen the structure to make it better able
desirable only where very low permeability is required,
to resist wave or ship loadings, superimposed dead
such as in the core of an earth dam. In such cases
loads, etc.
precautions, such as the construction of inverted filters
or sealing of structural joints, etc., are necessary to
Cavities or voids in the soil structure may be
prevent the erosion of fines. Since surface water (such
grouted using sand/water mixtures, portland cement,
as rainfall) can contribute greatly to erosion, provision
clay, chemical grouts, or a combination of these
should be made for the disposal of runoff. Where
materials. Sand/water mixtures are applicable only
materials consist of the very erodable silts or fine sands,
where large cavities are present and the paths of soil
the structure must be sealed to prevent any influx of
loss have been sealed off. Cement grout is not
surface water.
considered applicable if the effective grain size of the in-
6.4.3 DEWATERING. Where excavation and
replacement of soil takes place below the water table, it
may be necessary to dewater the site by use of seepage
6-4
place soils, D10 (the sieve size through which only 10%
solidifies and impermeabilizes the soil, but it is
of the soil would pass), is less than 0.5 mm for loose
extremely expensive. Mixtures of cement and clay are
soils and 1.4 mm for dense soils. Thus, portland
also used, sometimes with the addition of a chemical
cement grout is most applicable to the situation where
deflocculent. One of the more recently developed
the grout can be pumped directly into cavities.
chemical grouts polymerizes in the soil voids; however,
it is also expensive. With fine-grained backfills, grouting
An effective grouting procedure for sandy
is generally of no benefit except to fill cavities or to seal
materials consists of injecting solutions of sodium
off paths of soil removal.
silicate and calcium chloride. This procedure both
6-5
CHAPTER 7 - STEEL STRUCTURES
SECTION 1. CORROSION
Structural steel is used in most metal waterfront
requires an electrolyte or current-carrying medium
structures because it is strong, readily available, easily
between different parts of the corrosion cell. In marine
fabricated, and not excessively costly. Cast or
submerged areas, seawater is the electrolyte; in marine
fabricated steel is normally used for such accessories as
atmospheric areas, salt spray provides the electrolyte.
bitts, bollards, cleats, and chocks. Other metals have
Oxygen greatly accelerates corrosion, which accounts
specialized uses because of unique properties, such as
for the rapid corrosion that takes place in the splash-
being lightweight (e.g., aluminum) or corrosion resistant
zone area of waterfront structures where both seawater
(e.g., monel).
and oxygen are in abundance. Like most spontaneous
chemical reactions, corrosion increases with increasing
7.1.1 DEFINITION OF CORROSION . Corrosion is the
temperature.
destruction of a metal by its reaction with the
environment. This reaction is an electrochemical
7.1.2 TYPES OF MARINE CORROSION . There are
oxidation process that usually produces rust or other
many types of marine corrosion that can occur to steel
metal oxide. A more lengthy description of corrosion
waterfront structures and many methods for corrosion
and the corrosion process can be found in References
control. In actual practice, these methods are combined
7-1 and 7-2.
in an overall corrosion control program. The most
common types of metal corrosion occurring in a marine
Since corrosion is an electrochemical process, it
environment are described in Table 7-1.
SECTION 2. PROTECTIVE COATINGS
7.2.1 PROTECTION. The chief means by which
inhibitive pigments (e.g., chromate salts and red lead)
protective coatings impart protection to steel is by
when properly formulated in a primer pigment can deter
providing a barrier between the metal and the
corrosion should there be a break in the coating barrier.
environment that is necessary for corrosion to occur. In
all cases the coating must be free of pinholes or other
Reference 7-3 presents a comprehensive
discontinuities and of sufficient thickness to prevent the
coverage of paints and protective coatings.
environment from reaching the metal. Certain corrosion
7-1
Table 7-1. Types of Corrosion
Type
Description
Remarks
Galvanic Corrosion
Two dissimilar metals connected to each
1. New steel is anodic to old steel.
other electrically in an electrolyte (e.g.,
2. Brightly cut surfaces (e.g., pipe
seawater). Current flows through the
threads) are anodic to uncut
electrolyte from the more reactive
surfaces.
metal (the anode) to the less reactive
3. Steel is anodic to its surface mill
metal (the cathode), thereby corroding
scale.
the anode area while protecting the
4. Highly stressed areas (e.g., pipe
cathode area from corrosion,
bends) are anodic to less stressed areas.
Stray Current
Occurs on metal surfaces wherever stray
Stray current corrosion should always
direct current passes from them to an
be suspected as the cause of accelera-
electrolyte. This current most fre-
ted corrosion in areas adjacent to
quently arises from electric railway
sources of DC current and checked
and crane systems, improperly
for by detection of current flow.
grounded welding generators, and adjacent
cathodic protection systems.
Differential
Occurs from differences in chemical
Corrosion occurs in area of lower
Environmental
composition of the medium. Usually
oxygen content. On steel piling, this
results from different levels of
is just below the mean low tide level.
aeration (oxygen content); less fre-
Also in crevices and corners because
quently from different salinities
less oxygen is there.
Erosion-Corrosion
Scouring action of sand and other
1. Commonly found at or just above
abrasives exposes bright metal and
the mud line on steel piling or riser
keeps the corrosion active
chains of moorings.
2. Wind in sandy areas.
Biological Corrosion
Marine biological organisms accel-
1. Organisms may create different oxy-
erate corrosion by changing the
gen levels in the electrolyte.
normal environment
2. Organisms may create corrosive products
through their metabolism or decomposition.
3. Organisms may remove the protective film
of corrosion products from metal surfaces.
7-2
7.2.2 SURFACE PREPARATION. The proper
Structures Painting Council Paint Specification No. 16
preparation of steel surfaces prior to coating is essential
[7-6] have had many years of excellent service.
for maximum coating life and protection of the steel.
Epoxypolyamide coatings, such as MIL-P-24441, have
The selection of the type of surface preparation depends
provided many years of excellent protection to steel
upon the type of paint used, the condition of the surface
waterfront structures, too. Zinc inorganic coatings, such
to be painted, economic considerations, and such
as in Class 3 of MILP-23236, have been used
practical limitations as time, location, accessibility, and
successfully on the atmospheric portions of steel
availability of equipment. Standards for surface
offshore platforms and waterfront structures [7-7]. They
preparation are found in References 7-4 and 7-5.
must be top-coated with an organic coating for long-term
performance in seawater. Vinyl resin paints, such as
Dry abrasive blasting of steel is generally
VR-3 and VR-6 of the Bureau of Reclamation, perform
necessary for optimum performance of modern
well on steel. Petrolatum-coated tapes [7-8] have been
synthetic coatings. It removes mill scale, rust, corrosive
used successfully to protect steel utility lines under
salts, and other foreign matter that is detrimental to
piers. They can be applied to wire-brushed as well as
good coating application and imparts an anchor pattern
abrasive-blasted surfaces. The encapsulation of steel
(tooth) to the steel to which the coating can bond tightly.
piling with PVC wraps has been reported [7-9] to impart
long-term protection from corrosion by producing an
Many local air pollution control agencies may
oxygen deficient environment.
restrict dry abrasive blasting because of the dust emitted
during operation. The addition of water to the abrasive
7.2.4 COATING APPLICATION . In a marine
may be necessary to meet the air pollution control
atmosphere, conventional spray, roller, or brush
regulations. In such an event, a rust inhibitor needs to
application may be appropriate, as recommended by the
be added to the water to prevent rusting before paint
coating specification or supplier. A coal tar coating is an
application.
economical choice in an atmospheric area where the
black color is acceptable.
Waterblasting of steel without an abrasive may
provide a clean surface for re-painting without requiring
Application of coatings between tides may be
an abrasive-blast finish. Wire brushing, either manually
made by spray, roller, or brush, but the coating must be
or with power equipment, can in some cases provide an
one that will be unaffected by water and will cure under
adequate steel surface for painting.
it. Steel Structures Painting Council Paint Specification
No. 16 and MIL-P-24441 are two such coatings.
7.2.3 RECOMMENDED COATINGS. Coal tar coatings
are frequently used on steel waterfront structures.
Two different types of coatings are available for
Since they are relatively soft, their effectiveness in
application underwater [7-10]. One is a thick putty-like
waters where barnacle fouling is heavy may be limited.
material that is applied by the palm of the hand, and the
Coal tar epoxy coatings are tough, durable, and very
impermeable to water; thus, such coatings as Steel
7-3
other is a lower viscosity material that can be applied by
thickness of protective coatings is very important
brush or roller.
because it is directly related to barrier protection.
Measurement of both wet and dry film thickness is
7.2.5 INSPECTION OF COATING . Protective coatings
described in CEL Techdata Sheet 74-11 [7-12].
should be inspected at the time of application to make
certain that all application requirements have been met.
Coatings on metal surfaces should be checked
Appendix A of Reference 7-11 is a general guide to
for holidays, pinholes, or other discontinuities using one
inspection of facilities maintenance painting. Visual
of the commercially available holiday detectors. A low-
inspection of coatings before, during, and after
voltage detector (e.g., 50 to 60 volts) is ordinarily used
application can be effective in detecting deficiencies in
on thin films (i.e., 12 mils or less), and a high-voltage
the materials, surface preparation, or coating
detector (e.g., 10,000 volts) on thicker coatings. The
application. If deficiencies in the coating material are
coating must be free of water before the detector is
suspected, then laboratory testing should be done on
used. Any localized damage to the coating by a high-
paint from unopened cans to verify them. The film
voltage detector must be repaired.
SECTION 3. CATHODIC PROTECTION
7.3.1 DEFINITION. Cathodic protection is a system for
external power supply, but incorporates anodes of a
controlling corrosion of a metal surface by passing
special alloy that generate the necessary direct current
sufficient direct current onto it to make it a cathode, thus
by virtue of a natural voltage difference from the
eliminating the possibility of anodic loss of metal. The
protected structure (Figure 7-1). The galvanic anodes
electrolyte for cathodic protection is usually soil or
(also known as "sacrificial") are consumed, like the
water. References 7-13 and 7-14 present a detailed
anodes in a typical galvanic corrosion cell, in the
description of cathodic protection of buried structures.
process of generating current and, thus, have a limited
Thus, discussion of cathodic protection in the present
service life. The galvanic anodes are fabricated from
manual will be largely limited to structures in water. In
active metals and alloys; three basic materials are used
the maintenance of waterfront structures it must be
magnesium, zinc, and aluminum of high purity or other
remembered that cathodic protection can prevent
special composition.
corrosion of a new structure or stop corrosion on an
existing structure, but it cannot replace metal lost by
The impressed current system utilizes low-
corrosion of an existing structure.
voltage, high-amperage, direct current from an external
power source (Figure 7-2). The positive terminal of the
7.3.2 TYPES OF SYSTEMS. There are two basic
power source must be connected to the anodes, and the
systems for supplying the necessary direct current
electrical energy to a structure to cause it to become a
cathode. The galvanic anode system requires no
7-4
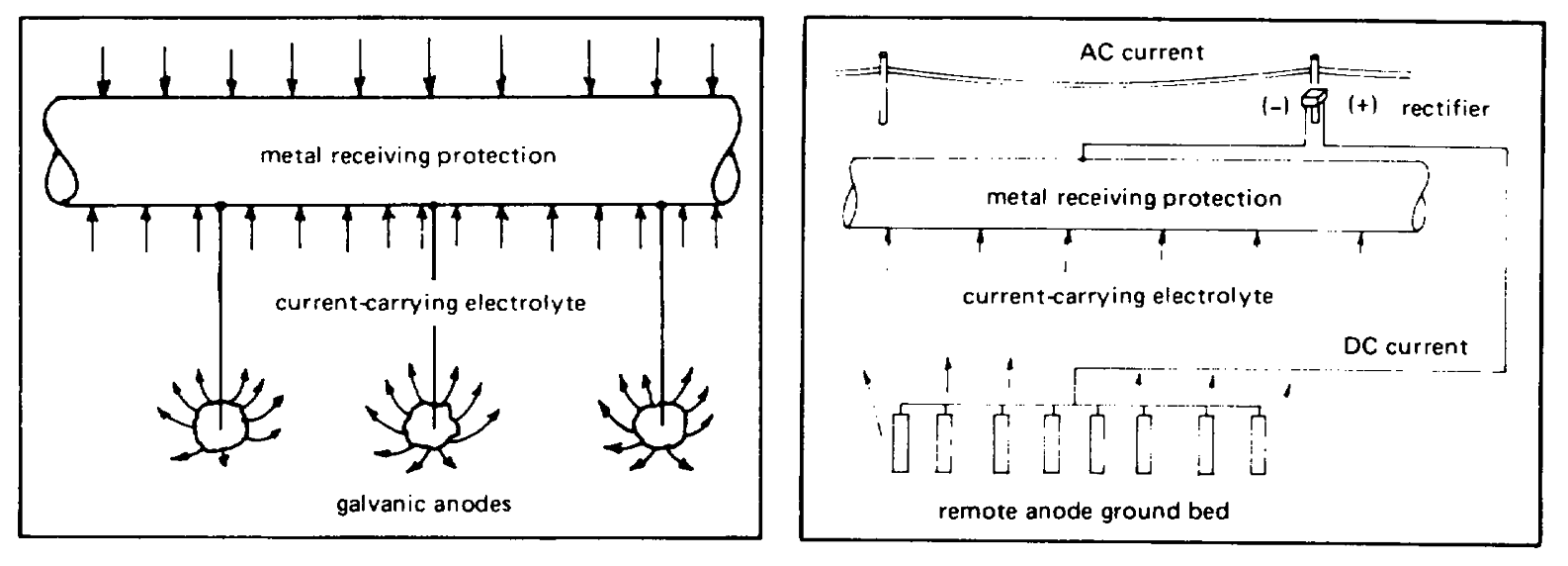
Figure 7-1. Galvanic system of cathodic
alloy,
protection.
Figure 7-2. Impressed current system of cathodic
protection.
negative terminal to the structure to be protected. The
relatively stable anodes used to discharge current have
platinized titanium alloy and platinized tantalum alloy
much longer service lives than galvanic anodes. These
are some of the materials used for the anodes.
anodes can theoretically he made from any electrically
Normally, rectifiers are used to supply the DC power to
conductive material. However, unless the material is
the system using available AC shore power. Sections in
inert in the environment, it will be consumed. Scrap
References 7-1, 7-13, and 7-14 discuss the selection of
iron, special lead alloys, platinum, platinum-palladium
and requirements for cathodic protection rectifiers.
SECTION 4. SUBSTITUTE MATERIALS FOR STEEL
7.4.1 ALLOYS. There are many alloys which, if used
(100-ksi yield) are known to be immune to all forms of
properly, are more resistant to corrosion by seawater
attack in seawater and marine atmospheres at
and marine atmospheres. These alloys exhibit three
temperatures below 1500F. These alloys are high in
types of corrosion behavior. Some are essentially
cost and difficult to fabricate. They are, however,
immune to corrosion, while some corrode but at rates
relatively high in strength and low in weight.
significantly slower than steel. Some of these alloys are
essentially corrosion free if properly used, but may
7.4.1.2 Nickel Alloys. Nickel alloys, depending on their
corrode at extremely rapid rates if used improperly.
composition, can exhibit either totally immune behavior
or can be essentially immune when used properly.
7.4.1.1 Titanium Alloys . The titanium alloys are
Inconel alloy 625 and Hastelloy alloy C are essentially
essentially free from corrosion except for stress-
immune to corrosion in marine environments.
corrosion cracking in some alloys. The chemically pure
grades and the heat-treatable alloy 6A1-4V annealed
7-5




